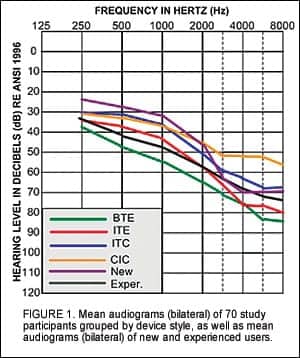
Evidence-based design is an essential component of technology that is aimed at enhancing audibility and improving communication for individuals with hearing impairment. Research conducted during product development enables safe and reliable technology, and also ensures that the product offers measurable advantage to the end user. Formalized rigorous testing prior to market release is essential for any manufacturer committed to a data-driven approach to product development.
Hearing technology developed at Starkey Laboratories Inc involves contribution from more than 350 audiologists, DSP engineers, psychoacousticians, and mechanical engineers over several years prior to release of a product. This thorough course of evaluation includes:
1) Internal benchmarking;
2) Algorithm research;
3) Formalized in-house alpha testing, and
4) External beta clinical trials.
This article describes the beta clinical trial of nFusion, which refers to the combination of the Destiny hearing instrument line with the Inspire OS programming software.
Evidence-Based Design
The Destiny 1200 hearing instrument was designed to address the most common areas of dissatisfaction reported by hearing aid users.1 Some of the primary goals included increased audibility of speech in noise and comfort in noisy “speech-less” environments, comfort for loud sounds, and a virtually feedback-free hearing aid fitting.
Several unique signal processing schemes were developed to provide higher usable stable gain, gain adaptation for differing acoustic environments, and seamless adaptive switching between omni-directional and directional microphone modes. The following are descriptions of the various algorithms in the new products.
Feedback cancellation. Active Feedback Intercept (AFI) is a new feedback cancellation algorithm that uses a multiband approach to adaptively control feedback, adjust to changes in the feedback path, and prevent entrainment artifacts. The acoustic feedback path can be precisely mapped in the clinic using the initialization process. AFI allows the instrument to provide a higher degree of usable stable gain to maximize speech intelligibility while virtually eliminating excessive feedback for the user.2,3
Speech and comfort enhancement/noise reduction. Acoustic Signature (AS) is an algorithm that uses the principles of pattern recognition to classify sounds arriving at the microphone(s) of the hearing aid and automatically apply that knowledge.4 Based on its features, the sound is classified into one of five categories: quiet, speech, wind, mechanical sounds, or other sounds. These categories were targeted because they represent environments in which hearing aids users are often dissatisfied with their devices. Following classification, gain adaptation is applied to maintain user comfort.
Adaptive directionality. Directional Speech Detector (DSD) is an algorithm that switches seamlessly between omni-directional and fixed directional microphone modes. The automatic switching logic maximizes directional benefit in adverse listening conditions, eliminating the need for the user to manually make this change. The decision to switch between omni-directional and directional modes is governed by the input level, signal-to-noise ratio (SNR), and the relative outputs of the two microphone modes.
Telephone/cellular phone use. The new line of products is equipped with a switching mechanism designed to activate the Automatic Telephone Response (ATR). This allows the hearing aid to automatically switch to a memory dedicated for phone usage—via the induction coil or an acoustic response—when a telephone receiver is placed next to the hearing aid. Although ATR is not new to the industry, the use of nanotechnology has allowed for greater switching success across all device styles without the need, in most cases, for an external magnet.
Large-Scale Beta Clinical Trial
Twelve external sites—including three major universities (Northwestern University, Washington University, and Utah State University), two medical centers, and seven private clinics—tested the new hearing device on 70 patients.
Objectives. The broad objectives for this clinical trial were to measure participant benefit from the Destiny 1200 and to evaluate the functionality of Inspire OS fitting software. Specific goals were to: 1) Assess the adequacy of “Best Fit” as a starting point for fittings; 2) Appraise the success of AFI in reducing the occurrence of feedback; 3) Evaluate the switching success of the ATR; 4) Rate satisfaction with the default gain adaptation provided by AS; 5) Determine if Destiny 1200 performance matched or exceeded performance of participants’ own devices, and 6) Garner hearing professionals’ impressions of Inspire OS.

Methods
Participants were fitted using the “Best-Fit” NAL-NL1* fitting formula, a proprietary modification of the NAL-NL15 prescription designed to provide less high-frequency gain in accordance with findings by Smeds.6 Each multi-memory test device was programmed with the “Normal” (eg, the Best-Fit modified NAL-NL1) setting in Memory 1, in which all adaptive algorithms (AS, DSD, and AFI) were turned on. Memory 2 was programmed with an ATR for use on the phone.
During the first session, participants completed the Abbreviated Profile of Hearing Aid Benefit (APHAB)7 for their own hearing aids. They were provided with a customized questionnaire to complete in the field comparing the new Destiny 1200 devices to their own hearing aids.
Participants returned in 2 weeks for a second session. Their hearing instruments were fine-tuned, and speech-in-noise testing with the QuickSIN8 was completed in three conditions: unaided, aided with their own devices, and with the new devices.
After an additional 2-week period, participants returned for their final session. During the last session, participants completed the APHAB for the new devices, as well as supplementary custom questionnaires. Clinicians also completed an assessment of physical fit and cosmetics of the new devices, and evaluated the user interface and functionality of Inspire OS fitting software.
Results
Best Fit settings. The purpose of a Best Fit is to provide the ideal combination of settings that will be satisfactory to most patients, with minimal fine tuning adjustments for a few individuals. Participating sites adjusted the gain and frequency response of the new devices based on clinical experience, probe microphone measurements, and patient comments. To determine the adequacy of the prescribed gain, the Best Fit settings in Inspire OS were compared to the settings used at the end of the study (eg, Final Fit).

Figure 2 displays the difference between Best Fit and Final Fit for a 70 dBSPL input for all device styles. On average, gain for BTE devices was increased between 5 dB to 8 dB across the frequency range, while CIC devices demonstrated an increase in gain of the same amount primarily in the mid to high frequency range.
Post-hoc analysis of the data revealed an error in the gain being applied for the CIC model. Specifically, the modified NAL-NL1 provided approximately 3 dB less gain than intended at 1,000 Hz and up to 6 dB less at 3,000 Hz. This discrepancy explains the findings and has been corrected in the released software. The adjustments applied to Best Fit for ITEs and ITCs were negligible.
Active Feedback Intercept. Acoustic feedback was assessed at various stages throughout the beta clinical trial. During the first session, the presence of feedback was evaluated by the clinician and participant with AFI set to adaptive. In an attempt to induce feedback, participants were asked to perform a number of exaggerated motions, including yaw, pitch, and roll head movements, as well as opening and closing the jaw. Under these conditions, feedback was found to occur in only 1 out of 140 devices. Initialization of AFI eliminated feedback for this sole participant.
Participants were also asked to report any instance of feedback while using the devices in the field in order to evaluate the performance of AFI under realistic conditions. Reports indicated that 6 out of 136 (4%) devices had some type of feedback while in general usage. Feedback during telephone use was reported by 18 out of 70 (26%) participants. Of those 18 participants, 11 described the feedback as either “Slight, but OK” or “Occasional, but OK,” meaning they would experience brief tonal feedback while AFI adapted to the change in feedback path.
Automatic Telephone Response. Participants were asked to rate the effectiveness of the ATR using a variety of telephones, including hard-wired and cordless land line telephones and cellular models. Results indicated that the test devices consistently switched for 77% of participants, across all telephone styles. Using a magnet in conjunction with a particular telephone increased the switching success rate to 100%. Additionally, 80% of respondents (41 of 51 subjects) indicated that they liked having an automatic switching feature for telephone use.

Acoustic Signature. Field ratings relating to AS were obtained after participants had worn the Destiny 1200 devices for 2 weeks. All users were asked to rate the overall listening comfort in noisy environments with the devices. Figure 3 indicates that 84% of all participants rated their comfort level as “OK,” “Good,” or “Very Good.” When asked about their preference for the amount of gain adaptation, the majority of the participants reported the level of the noise in various environments to be “Just Right” (Figure 4), indicating that the default settings were appropriate.

Participants were also asked to rate their ability to understand speech. In listening environments that involved smaller groups of people, 94% of the participants believed their communication ability was “OK,” “Good” or “Very Good” with the new instruments while only 6% rated communication as “Poor” (Figure 5). As expected, the ratings in larger group settings were poorer, with only 78% indicating that their communication ability was “OK” or better.


Comparison Measurements of New vs Own Devices
Objective SNR measurements. Objective speech-in-noise evaluations were performed using a modified QuickSIN test.8 The test was administered via the Surround Town setup (Figure 6), a 5.1 surround sound speaker configuration that is used as a component of the Inspire OS fitting software.9
Although Surround Town is actually a virtual reality fitting tool, it provided a means to create a relatively diffuse soundfield for the QuickSIN test. The original QuickSIN recording was modified such that the target sentences were delivered from the front speaker and uncorrelated babble was presented from the remaining four speakers. The QuickSIN test was then administered as per the standard protocol with speech at 70 dBSPL and the SNR decreasing from +25 to 0 dB over the course of a single list.
Participants were tested unaided, with their own devices and with the Destiny 1200 devices. All directional Destiny 1200 devices were programmed with static directionality for this measure, and all other algorithms remained active including AS and AFI. Three lists were administered, and the scores were averaged to obtain the SNR loss in each condition. The SNR loss represents the dB level at which the participant requires speech to be above the noise floor in order to repeat the sentence with 50% accuracy.10

Figure 7 illustrates the average SNR loss in each condition for experienced and new hearing aid users. It should be noted that higher values of SNR loss indicate poorer performance. As expected, performance in the unaided condition is significantly worse than in the aided condition for both groups (p<0.05). Results also indicate that participants did not perform significantly better with their own devices over the unaided condition. Additionally, experienced users demonstrated an average improvement in SNR loss of 1.3 dB with the new devices compared to performance with their own devices; this average includes both directional and omni-directional devices.

Subjective hearing aid benefit measurements. The APHAB is a standardized self-assessment tool that measures perceived benefit in four main categories: Ease of Communication, Effect of Reverberation, Effect of Background Noise, and Aversiveness of Sounds. Figure 8 illustrates the average benefit reported by all experienced users of amplification for their own devices and the new devices. Results indicated a statistically significant advantage for Destiny 1200 in terms of ease of communication and in background noise (p<0.05). Further, there was a slight advantage for the new device in reverberation and aversiveness of sounds.
Experienced users were also asked to compare communication ability with Destiny 1200 to their own devices in a given listening environment. Results indicate that less than 10% of experienced users preferred their own devices over the test devices (Figure 9).

Clinicians’ Rating of New Software
Of particular interest to our company was how the new software’s “look and feel” would be received by the hearing health care community. To garner this information, sites were asked to rate several aspects of Inspire OS fitting software in relationship to their usefulness in fitting hearing aids and addressing needs of the participants. Using a 5-point scale where 1 equaled “Not Useful” and 5 equaled “Very Useful,” clinicians rated the speed of the software, software readiness (being bug-free), navigational features, complexity of the software, and the flexibility of Inspire OS user interface. Average responses in each category were between 4 and 5 (Figure 10), indicating clinicians found the fitting tools and overall functionality of Inspire OS to be very useful.

Discussion and Conclusions
The purpose of a large scale beta clinical trial is to acquire relevant information about a product offering that can be used to enhance the end result. Research of this nature provides a data-based reference for decisions regarding refinements.
The evaluation of nFusion revealed that:
1) The new hearing device (Destiny 1200) provides measurable subjective and objective advantage, even when compared to other hearing instruments.
2) The new device offers increased speech understanding in noise while maintaining listening comfort in a variety of listening environments.
3) The Inspire OS fitting software is intuitive to use and provides the necessary control and flexibility for addressing individual needs.
Clinical evaluation of new technology is an integral part of the research and development process at Starkey Laboratories Inc. Ongoing communication with and contribution from hearing health care professionals ensures a continuous improvement of the patient journey.
Acknowledgement
The authors thank Mead Killion and Patty Niquette of Etymotic Research, Elk Grove Village, Ill, for their guidance and contribution of the QuickSIN test for our specific research purposes.
References
1. Kochkin S. MarkeTrak VI: 10-year customer satisfaction trends in the US hearing instrument market. The Hearing Review. 2002; 9(10): 14-25,46.
2. Banerjee S, Recker K, Paumen A. A tale of two feedback cancellers. The Hearing Review. 2006;13(7):40-44.
3. Merks I, Banerjee S, Trine T. Assessing the effectiveness of feedback cancellers in hearing aids. The Hearing Review. 2006;13(4): 53-57.
4. Banerjee S, Olson L, Recker K, Pisa J. The truth about acoustic signature. Hear Jour. In press.
5. Dillon H, Katsch R, Byrne D, Ching T, Keidser G, Brewer S. The NAL-NL1 prescription procedure for non-linear hearing aids. In: National Acoustics Laboratory Annual Report. Sydney, Australia: NAL;1997: 4-7.
6. Smeds K. Is normal or less than normal overall loudness preferred by first-time hearing aid users? Ear Hear. 2004;25(2): 159-172.
7. Cox RM, Alexander GC. The abbreviated profile of hearing aid benefit. Ear Hear. 1995;16(2),176-186.
8. Killion MC, Niquette PA, Gudmundsen GI, Revit LJ, Banerjee S. Development of a quick speech-in-noise test for measuring signal-to-noise ratio loss in normal-hearing and hearing-impaired listeners. J Acoust Soc Am. 2004;116(4(Pt. 1)):2395-2405.
9. Howes C, Olson L. The role of virtual reality in hearing instrument fittings. The Hearing Review. 2006;13(5):60-63.
10. Killion MC. SNR loss: “I can hear what people say but I can’t understand them.” The Hearing Review. 1997;4(12):8-14.
This article was submitted to HR by Laurel Olson, MA, manager of clinical product research, and Justyn Pisa, AuD, research audiologist for Starkey Laboratories Inc. Correspondence can be directed to Laurel Olson, Starkey Laboratories Inc, 6600 Washington Ave S, Eden Prairie, MN 55344; e-mail: [email protected].