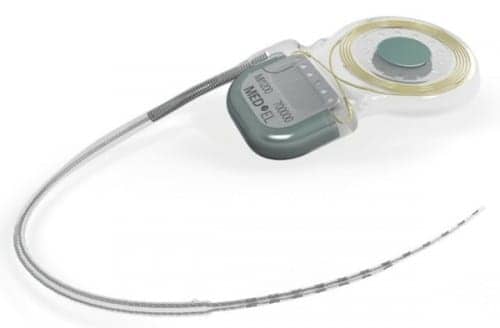
MED-EL USA, Durham, NC, has announced that its Synchrony cochlear implant (CI) has successfully been implanted in the first US patient at the University of North Carolina Hospitals. As reported in a January 23, 2015 article in The Hearing Review, the Synchrony CI was FDA-approved for MRI without requiring surgical removal of the device’s internal magnet. According to MED-EL’s announcement, the first US recipient of the Synchrony CI is an 83-year-old patient with bilateral moderately-severe to profound hearing loss who also has a history of back surgery and a likelihood of future 3.0 Tesla (T) Magnetic Resonance Imaging (MRI) examinations to monitor his back condition.
“The upper age limit of cochlear implant candidates continues to rise,” said Harold Pillsbury, MD, chair, Department of Otolaryngology/Head and Neck Surgery, University of North Carolina School of Medicine. “With age comes an increased risk for diseases that rely on the latest diagnostic technology, like 3.0 T MRI. UNC is proud to be the first facility in the country to offer Synchrony’s cutting-edge cochlear implant technology.”
MED-EL reports that the commercial availability of the Synchrony CI will be announced in the coming weeks. The company says the implant’s magnet design is approved for 3.0 T MRI examinations which allow imaging of organs and tissues at higher resolution. MRI usage is reportedly on the rise, at a rate of approximately 10% per year, so this implant’s magnet design is said to be an important advancement.
The magnet of a cochlear implant device holds the external audio processor in place, according to MED-EL, and previous magnet designs are only robust against MRI field strength of up to 1.5 T. Synchrony’s diametrically aligned magnet freely rotates within its titanium housing and aligns itself with the magnetic field in the MRI tube. Due to the self-alignment feature, there is no risk in a loss of implant magnet strength during MRI scans and no torque acts on the magnet in the MRI tube.
“Providing the sense of hearing, together with the peace of mind knowing that future MRIs are possible without the need for additional surgery, is a major quality-of-life advancement and MED-EL is proud to be the leader in making this technology available,” said Raymond Gamble, president and CEO, MED-EL North America.
Source: MED-EL