Research scientists at the Karolinska Institutet in Sweden are trying to identify the molecules behind tinnitus in an effort to find a treatment to silence the ringing and other phantom noises that many people experience.
Ringing in the ears or hearing other sound inside one’s head when no external sound is actually present is known as tinnitus. It’s a common problem that affects more than 1 person in every 10. While many learn to live with it, tinnitus can become much more than a minor ringing or buzzing sound. It can seriously disturb sleep, concentration and daily living to the point of causing depression and anxiety.
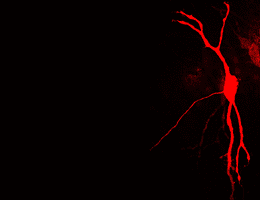
“Our study in mice proposes the first gene related to tinnitus,” says Christopher R. Cederroth, PhD, an assistant professor at Karolinska Institutet. He and his team discovered that without normal function of the GLAST gene, animals are more prone to develop tinnitus. The gene codes for a glutamate transporter, a protein that works to take the neurotransmitter glutamate away from the synaptic space, where neural communication occurs, back into the cells. An interview with Dr Cederroth about this research is available on video.

Interview with Professor Christopher R. Cederroth, Karolinska Institutet, on tinnitus (Video: Frontiers of Behavioral Science; Karolinska Inst.)
“Removing excess glutamate from the synapse is vital for healthy neural function, as uncontrolled glutamate levels outside neural cells overexcite neurons and have toxic effects,” explains Cederroth. “Dysfunctions in glutamate transporters have been previously associated with seizures and amyotrophic lateral sclerosis–and now, tinnitus.”
The results, published in an August 2016 edition of Frontiers in Behavioral Neuroscience, point beyond the known predominance of GLAST in the cerebral cortex and hippocampus, as its protective effects could occur in the inner ear. “Our study opens the possibility that higher expression of GLAST in the cochleae of mice could create the resistance to auditory insults, by preventing ototoxicity.”
Since his first tinnitus studies in mice in 2009, Cederroth saw the need to improve the detection of tinnitus in this species, which offers facilitated behavioral and genetic manipulations for our understanding of the mechanisms behind the auditory disorder.
“Curiously, mice appear to be resistant to developing tinnitus,” says Cederroth. “Only 20-50% of mice show behavioral changes that point to tinnitus after known risk factors, compared to 70-75% of rats, for example.”
This finding posed a technical problem to scientists like Cederroth, who are looking for biological causes of the disturbance. Testing behavioral detection of a sound gap, in different mouse strains, turned out to be a smart idea. Cederroth and his team found a solution that involved identifying the right mouse strain and the right stimulus timing. This improved the range of responses they could detect from animals with tinnitus in a mouse strain that is widely used in other fields for genetic testing, giving the entire scientific community a robust model for further testing of biological mechanisms of tinnitus.
This is seen as a big step forward. Each mouse strain has a certain genetic background and shows a different level of baseline sensitivity to silent gaps. So modifying a certain gene in a sensitive strain and being able to observe a range of behavioral functions can point to the genes, and related molecules, that are required for specific types of tinnitus. Cederroth’s team found that when the GLAST gene was knocked-out of mice with the C57 genetic background, the animals developed more tinnitus when exposed to salicylate, a derivative of aspirin known to be transiently toxic to ear cells.
Experimental models of tinnitus are normally made by exposing animals to loud noise or to drugs toxic to ear cells, such as salicylate, and then presenting animals with various behavioral tasks to measure their perception of non-existing sounds. One such behavioral test is GPIAS, which stands for gap pre-pulse of the acoustic startle reflex. The principle behind this task is the normal and involuntary ability of animals to suppress a startle reflex in response to a loud pulse, if the startling pulse is preceded by a silent gap in the background noise. The pre-pulse gap normally serves to prepare the animal for the upcoming startling pulse, such that they startle less.
“An animal that does not show this normal behavioral inhibition with the pre-pulse gap is showing a less efficient gap detection, in the form of a greater reflex response,” explains Cederroth. “This could happen when the animal perceives a phantom sound, like tinnitus, interfering with the ability of sensing the gap properly,” explains Cederroth. “As we change the frequency of the background noise to closely match an animal’s ringing in the ear, the startle reflex is less efficiently suppressed by the pre-pulse gap.”
Because of the improved sensitivity of the behavioral task in detecting tinnitus and clear candidate molecules, the GLAST deficiency model may very well serve to distinguish more subtle, yet unidentified mechanisms on how tinnitus is triggered and maintained. The researchers say they now need to investigate in more detail what happens in the ear and in the brain to identify where GLAST is acting and how.
“There is a current need to standardize tinnitus diagnosis and how treatments are assessed, and our work points to the value of changing timing and frequency parameters to better diagnose cases using this methodology,” says Cederroth. “These improvements will have to be tested in humans with tinnitus, as it is of utmost importance for drug discovery to have comparable read-outs between animal models and humans. A method that can objectively quantify a decrease in tinnitus perception after a given treatment would be a major breakthrough in the field. When coupled with human genetic studies, this endeavor could ultimately improve the drug development process and lead to the preventive or curative treatment of tinnitus.”
According to Cederroth, tinnitus is a symptom, not a disease itself, as it can result from various potential causes, such as hearing loss due to damage of hair cells in the ear, head injury or infection, toxic drugs, psychiatric and neurodegenerative disorders, or circulatory problems. He says that understanding tinnitus is a multi-disciplinary affair, and current knowledge is relatively scattered across disciplines.
“This work is part of an effort to gather all the expertise to solve problems of heterogeneity, in how tinnitus becomes a problem to some but not all people, and why they respond differently to treatments,” says Cederroth. By bringing together the global expertise in tinnitus research, he hopes awareness will increase. “People with tinnitus need help that until now, we have not been very successful at providing.”
Source: Frontiers in Behavioral Science






If the report was filed in 2016, and research began in 2009 (this study), wondering why there hasn’t been an update since then. Seems we have an almost 2 or 3 year gap since the main components of this study were forumlated. Has the research through this study stopped? Sure would like to see some steps toward reducing or eliminating the condition regardless of the cause. And if it is drugs, one that doesn’t carry bad side effects.
Hi to every one who suffers Tinnitus, as a sufferer since 2011 with constant ringing & hissing in both ears 24 hrs a day volume of the scales you all have 100% of my sympathy and best wishes on future health both medically and mentally.The medical world we live in today have absolutely no idea the impact that Tinnitus can have on 24 hrs a day on a persons life.The infliction is hardly regonised as a threatning problem .No sleep , No concentration ,No peace ,No cure No ,understanding , No escape , There is no unstanding that for a large number of suffers it will be the last thing they will ever hear .I hope please more unstanding ,more aknowlegedment,and more funding to end the The lonesome suffering which feels no one cares .God bless you all and good luck .
Don’t you guys get it. Were miserable and all we think about is death !!! Please !!! Please for the love of God help us already. We have families and kids and want to live happy again. I pray and I pray. Somebody has to come out with a cure. Something. Please help us. Thank you.
I pray that the good Lord helps us all with a cure or relief. Till then keep the faith and know that someone is going through the same thing as you are. May God give you strength to endurethis condition.
The article ends with “people need help with tinnitus that until now we have not been able to provide.” So, where is the help? I have the world’s loudest tinnitus and am desperate for someone somewhere to do something. Please God come out with something that will give me just a moment’s peace. Thank you.
My personal view is that all these doctors are incompetent, blaming depression as a cause of tinnitus or thinking a person’s ringing is harmless, so we can just live with it. The problem with this world is that not enough people have severe tinnitus. The research is not profitable and not a priority. No one can understand how bad it is unless they have it themselves.
Have suffered from tinnitus for many years. Has only gotten worse..can’t sleep. Doctor’s say there is no cure…any advise or new successful treatments ?
Andy Rutherford
I have 3 types of [tinnitus] sounds: whistling right ear, rotor blades left, and top central continuous music. When will human trials begin?
You just hear this and that like its working for mice and no solution . I don’t think anybody is interested in finding in right solution for the tinnitus . If government want to find solution for it they could have found it years ago bu investing some money .
In my opinion, there has been a dreadful lack of attention to the plight of those with tinnitus. However, I think the good news is that in recent years there have been great gains in our understanding of tinnitus and the provision of funding for studies involving tinnitus. As with hearing loss, I doubt there will ever be a “magic pill” that completely cures it, but I DO think there are some excellent recent advancements in both research and meaningful progress for people with tinnitus like the AAO-HNS practice guidelines.
There are so many people with this condition that if someone finds something to help us, will the National Health Service (NHS) be able to afford any treatments?
Great news, I have had this very distressing condition for eight years. It is usually brought on by over-exposure to loud noise.
Young people should be made aware of the consequences of listening to loud music at concerts and with home entertainment music equipment.