A look at seven chronic conditions associated with hearing loss—ranging from depression to diabetes—along with a review of some of the key supporting scientific evidence and papers.
In only the last dozen years, many important studies have surfaced linking hearing loss to disabling conditions, such as cognitive decline and Alzheimer’s disease, clinical depression, diabetes, falls among the elderly, heart disease, and many more. These linkages are often referred to as comorbidities, which can be defined as the simultaneous presence of two or more chronic conditions or diseases in a patient. A chronic condition can be defined as a health condition or disease that is persistent or otherwise long-lasting in its effects. The term chronic is often applied when the course of a disease lasts for more than 3 months. Under this definition, hearing loss clearly qualifies as a chronic condition.
In fact, hearing loss shares many of the same traits as other chronic diseases. For example, the chronic disease of diabetes is similar to hearing loss in that both conditions are invisible, progressive, painless, often incurable, but treatable. Both usually require “front-loaded” professional care—where the key counseling occurs in the first several clinical encounters—and both conditions require self-managed behavioral change for long-term success. Likewise, when properly managed, patients benefit from diabetes treatment or hearing loss remediation by regaining activities that were previously restricting their physical behavior and quality of life.
The following article focuses on the research literature surrounding seven comorbid conditions associated with hearing loss—social isolation and loneliness, depression, balance problems and falls, cardiovascular disease, diabetes, dementia, and even mortality. For many of these conditions there exist fairly extensive and interesting research. Thus, due to space restrictions, this article limits discussions to 1-3 papers per condition. (Editor’s note: A recent webinar by the author, sponsored by Hamilton CapTel, provides more details and can be accessed using the URL at the end of this article.)
Additionally, beyond the seven conditions noted above, there are other comorbidities linked to hearing loss, including but not limited to fibromyalgia, anemia, psoriasis, rheumatoid arthritis, kidney disease, and sleep apnea.
Prevalence of Hearing Loss and Models of Its Health Consequences
The extent of hearing loss in the United States is important to understand when placing comorbidities associated with hearing loss into context. Agrawal and colleagues1 used the 2001-2008 National Health and Nutritional Examination Surveys (NHANES) to show that the overall prevalence of hearing loss (?25 dB HL bilateral) was 26.8% for those between ages 60-69, 55.1% for people ages 70-79, and 79.1% for people ages 80 and older. Moreover, the rates becomes considerably higher when unilateral hearing loss is included (44.9%, 68.1%, and 89.1%, respectively). Therefore, hearing loss is a highly prevalent chronic condition, particularly in the senior adult population.
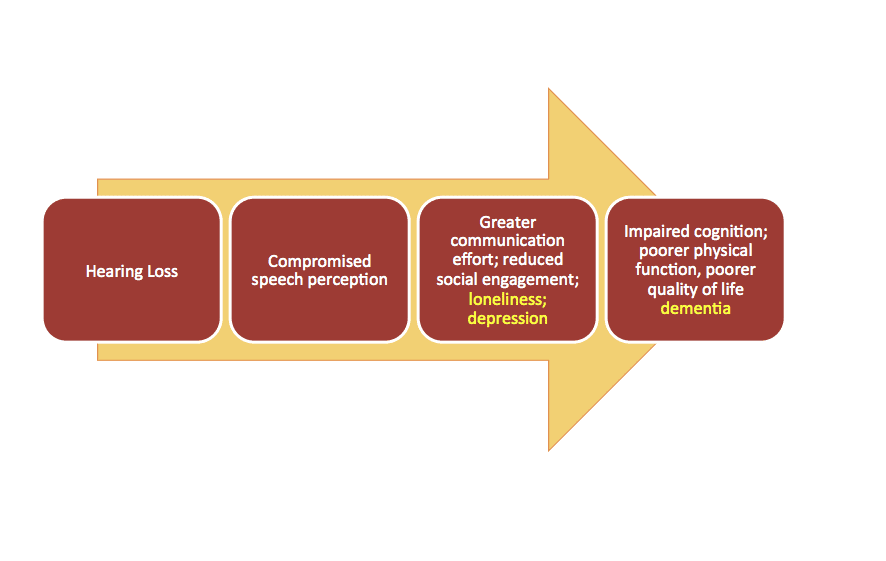
Most dispensing professionals recognize or may even intuitively understand the factors underlying the psychosocial consequences of hearing loss: social withdrawal and isolation, depression, reduced quality of life (QoL), and cognitive decline. A general model for these deleterious consequences is shown in Figure 1. The basic premise is that hearing loss can result in compromised speech comprehension, more difficult communication leading to embarrassing or mentally taxing situations, and ultimately cause the individual to withdraw from the social activities that characterize a healthy communicative lifestyle.
Thus, hearing loss may lead to the psychosocial consequences listed above, and perhaps concomitant reduced cognitive and physical function and QoL, as noted below.
7 Comorbidities Linked to Hearing Loss
1) Social isolation and loneliness. Although social isolation and loneliness are usually looked at as different constructs, for the purpose of this general discussion the two are combined here. One of the most striking studies on this topic was the 2016 research by Sung and colleagues2 at Johns Hopkins and the University of Oklahoma. They looked at 145 participants (ages 50-94) enrolled in the Studying Multiple Outcomes After Aural Rehabilitative Treatment (SMART) study from 2011-2013. The researchers measured loneliness using the UCLA Loneliness Scale, and found that younger-age hearing loss was significantly correlated with greater loneliness. Other depressive symptoms and hearing-related quality of life, communication difficulties, emotional well-being, and mental health as indicated on the 36-item Medical Outcomes Study Short-Form (SF-36) were also negatively correlated with loneliness.
Nancy Donovan and colleagues3 looked at the association between higher cortical amyloid plaques—which have been linked to Alzheimer’s disease (AD)—with loneliness in cognitively normal older adults. The researchers found that, after controlling for a number of possible confounding factors, higher amyloid burden was significantly associated with greater loneliness as measured by the UCLA Loneliness Scale. Those in the amyloid-positive group were 7.5 times as more likely to be classified as lonely than non-lonely.
So, given these two studies, it’s certainly possible there are links between hearing loss and loneliness, and between loneliness and cortical amyloid burden. However, the question researchers now wish to answer is could hearing loss be a contributing factor leading to elevated cortical amyloid burden?
2) Depression. Data from Mener et al4 looked at 1,029 individuals ages 70-79 from the 2005-2006 and 2009-2010 two-year cycles of the NHANES study. The subjects in this study were measured for depression using the Patient Health Questionnaire (PHQ-9), and the results indicated that the odds of a person self-reporting a depressive disorder were 1.5 greater per 25 dB of hearing loss in the better ear. Moreover, the odds of self-reporting any depressive symptom increased to 1.63 per 25 dB. The results indicated that hearing loss was independently associated with depression.
Similar findings were found in a 12-year review5 of the Taiwan National Health Insurance Research Database (NHIRD) which included 5,043 patients with sensorineural hearing loss (SNHL) and 20,172 patients without hearing loss. The analysis revealed that the risk of depression was higher in the hearing loss cohort, with the hazard ratio being 1.73—again suggesting that hearing loss is an independent risk factor associated with depression regardless of age, gender, and comorbidities.
Li and researchers6 at the NIH and NIDCD looked at 18,318 adults in the NHANES database from 2005-2010. This data is a bit more nuanced than the previous studies cited above, because the researchers were specifically interested in examining depression as a function of the severity of hearing loss. The results appear to suggest a direct relationship, with the prevalence of moderate-to-severe depression being 4.9% for people reporting excellent hearing, 7.1% for those with good hearing, and 11.4% for those reporting a little trouble or greater hearing loss. This study also contains some interesting data that shows how, as PTAs increase, the incidence of depression also increases. The trend appears to be more obvious for women, and the exceptions were people who reported themselves to be “deaf.”
3) Falls. Falls are the leading cause of fatal and non-fatal injuries among the elderly leading to significant health, social, economic, and emotional consequences. Falls often lead to fatal outcomes within the first 12 months of a fall with injury in the senior population.
Lin and Ferrucci7 examined the association between falls and hearing loss among 2,017 people ages 40-69 in NHANES participants from 2001-2004. They found a significant association, with 1.4-fold increased odds of reporting a fall in the previous year for every 10 dB of hearing loss. Adjusting for demographic, cardiovascular, and vestibular balance function did not substantially change the magnitude or significance of this association.
4) Cardiovascular disease. An interesting 2009 study by Friedland and colleagues8 published in Laryngoscope showed that the audiometric patterns—particularly low-frequency (sloping) and flat (strial) losses—were strongly correlated with cardiovascular disease. In fact, the researchers reported that patients with low-frequency hearing loss should be regarded as “at risk” for cardiovascular events, and appropriate medical referrals should be considered. One chart in the study suggests that about 85% of diagnosed strokes were associated with individuals who had flat or low-frequency sloping losses, and the authors posit that this may reflect either a common vascular pathology within the cerebrovascular system or a generalized vascular compromise effecting both hearing and cardiovascular structures.
5) Diabetes. An extremely interesting population study looking at the association between hearing loss and diabetes was conducted by Kathleen Bainbridge and colleagues,9 published in the July 2008 edition of the Annals of Internal Medicine. These researchers looked at 5,140 adult NHANES participants from 1999-2004. The researchers report that hearing impairment was found to be more prevalent among those participants with diabetes. Following multivariate analyses, they found that people with diabetes had significantly increased odds of hearing impairment in worse and better ears at all severity levels and frequencies.
Meta-analyses can often reveal effects that are obscured in individual studies, particularly for studies that have a small sample sizes. A systematic review by Horikawa et al10 used 13 eligible studies involving 20,194 participants and 7,377 individual cases. Consistent with the Bainbridge et al study above,9 their meta-analysis revealed that prevalence of hearing loss among those with diabetes was more than twice that than those without diabetes. The association between hearing loss and diabetes was actually stronger among those people younger than age 60, and was independent of gender or chronic exposure to noise.
In 2016, Kim et al11 published a prospective cohort study of over 253,000 adults with baseline normal hearing who were followed from 2002-2014. Among this large number of participants, the hazard ratio for developing hearing loss (adjusted for noise exposure, body mass index, smoking, alcohol use, and exercise) was 1.04 among those with pre-diabetes and 1.4 with diabetes. The authors posit that the vascular effects of diabetes damage the blood supply to the cochlea leading to sensorineural hearing loss. Specifically, high blood glucose levels may damage the vessels in the stria vascularis and nerves impacting the biochemistry and neural innervation of the cochlea.
6) Cognitive impairment and dementia. The relationship between hearing loss and dementia has probably received the most attention in the press of all the comorbidities reviewed here. Perhaps the landmark study that elevated the interest of healthcare professionals and the public about this topic was the paper by Dr Frank Lin and colleagues published in 2011.12 The researchers prospectively studied 639 individuals who underwent audiometric testing and were dementia-free in the Baltimore Longitudinal Study of Aging from 1990-1994. The participants were followed for a median period of just under 12 years, and during this period there were 58 cases of incident all-cause dementia diagnosed, of which 37 cases were Alzheimer’s disease (AD). The risk of these two conditions increased with the severity of baseline hearing loss. In fact, after adjusting for other factors, the results showed that, compared with normal hearing, the hazard ratio for incident all-cause dementia was 1.89 for mild hearing loss, 3.00 for moderate hearing loss, and 4.94 for severe hearing loss. The risk for AD also increased with baseline hearing loss by 1.2 per 10 dB of hearing loss.
In a similar prospective population study, Gurgel and colleagues13 from the University of Utah followed 4,463 subjects without dementia at baseline, 836 of whom had hearing loss. Consistent with the Lin et al study,12 the results indicated that hearing loss was an independent predictor of developing dementia and that hearing impairment may be a marker for cognitive dysfunction in adults ages 65 and older.
A prospective study by Fritze et al14 looked at an even larger population—154,783 people—aged 65 and older from claims data generated by the largest health insurer in Germany. They followed these people from 2006-2010, during which time 14,602 dementia diagnoses were made. Gender, age, and comorbidities were controlled as potential confounders. The results showed that patients with bilateral hearing impairment had higher risks for dementia incidence than those without hearing loss.
What might be responsible for the link between hearing loss and dementia? A model proposed by Wayne and Johnsrude15 theorizes an interaction between bottom-up processing and top-down processing, and these combine to create the perceptual difficulties, challenges to cognitive resources, negative psychosocial outcomes, and ultimately, impaired cognition. So, adding loneliness, depression, and dementia to the our previous model in Figure 1, the resulting chain of events could hypothetically resemble Figure 2.
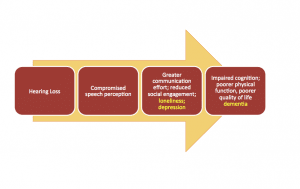
Figure 2. Example of a revised model from Figure 1 in which loneliness and depression, as a result of hearing loss and social limitations, contribute to cognitive decline.
7) Mortality. As if all these comorbidities are not enough, it turns out that there may even be a relationship between hearing loss and mortality! The Reykjavik study by Fisher et al16 examined the vision and hearing of 4,926 Icelandic individuals ages 67 years or older between 2002-2006 who were prospectively followed for mortality through 2009. In the study, individuals with hearing loss had a higher risk of death from all-cause cardiovascular disease than those without hearing loss.
Possible Effects of Amplification
Admittedly, all of the above is rather bleak. However, as any hearing care professional might suspect, it’s possible amplification could have some positive mitigating effects on several of these conditions. For example:
Falls. A study by Rumalla et al17 indicated that using hearing aids for 30 days may reduce the risk of falls. The experiment looked at the amount of time patients maintained stability when performing the Romberg foam and tandem tests in both the unaided and aided conditions. For many participants, the number of seconds the patients maintained stability significantly increased when using hearing aids. One possible explanation is that the brain uses sound cues to maintain spatial orientation, and is able to home in on spatial-orienting landmarks, just as visible objects serve as landmarks to improve stability with sight.
Depression and loneliness. With regard to hearing loss and depression, when we go back to the Mener et al4 study discussed earlier, the subjects’ odds ratio for being diagnosed with a major depressive disorder or any depressive symptoms were significantly lower for those wearing hearing aids than those who did not wear hearing aids.
Boi et al18 conducted a study involving 15 participants that supports the positive effects of hearing aids on reducing depression. In this study, it was shown that both depression in patients and caregiver burden were reduced during a 6-month period of hearing aid use. Additionally, there were changes in the Health Survey Short Form (SF-36) with particular improvements in the mental health and social functioning scores.
Audiologist Barbara Weinstein and colleagues19 have investigated the effects of hearing aids on loneliness. They were able to document a significant decrease in the perceptions of loneliness following 4-6 weeks of hearing aid use on both the total score and the Emotional Loneliness subscale score using the De Jong Gierveld Loneliness Scale.
Cognition. In terms of cognition and hearing aid use, Dawes and colleagues20 showed that hearing aid use had a positive effect on cognition which was independent of social isolation and depression. Similar results were found in a study by Acar et al,21 who found improvements in cognitive status after 3 months of hearing aid use as shown by scores on the Mini-Mental State Examination (MMSE).
MarkeTrak 9 (MT9) data,22 which was reported by the author and Janet Kihm, has also revealed self-reported psychosocial benefits of hearing aids. For example, hearing aid owners are less likely to say they have “little interest or pleasure in doing things” than non-owners or even those who did not report hearing difficulty. Hearing aid owners were less likely to report “feeling down, depressed, or hopeless” than non-owners or those reporting no hearing difficulty. MT9 data also indicated that hearing aid owners reported being less forgetful in the past year than non-owners.
What could explain the benefits of hearing aids on these psychosocial measures? The evidence is fairly compelling that the auditory system is neuro-physiologically plastic. For example, a 2008 study by Kevin Munro23 and a 2015 study by Limor et al24 make strong cases for auditory plasticity by examining changes in auditory processing tasks following hearing aid use. If we accept that the auditory system is plastic, we can envision how hearing aid use might influence the Wayne and Johnsrude model15 by providing enriched upstream auditory information to the brain, which in turn, could mitigate downstream consequences associated with increased cognitive load and other compromised psychosocial outcomes in the unaided condition.
Future Studies
The research evidence clearly points to associations between hearing loss and specific chronic conditions, as well as the potential positive impact of audiological rehabilitation. What is now critically needed are the initiation of well-designed randomized controlled studies that help us better understand the nature of these associations for the implementation of effective evidence-based interventions.
One large-scale study in the works is being led by Dr Lin at Johns Hopkins. The Aging and Cognitive Health Evaluation in Elders (ACHIEVE) study is designed to determine the effect of best practices and hearing rehabilitative treatment on rates of cognitive decline in 70-84 year-old well-functioning, cognitively normal adults with hearing loss. This study is investigating the mechanistic pathways for which hearing rehabilitation affects positive functioning.
Another example of an ambitious study is the SENSE-Cog Project in the European Union that looks at health for the eyes, ears, and mind in 7 countries. The principle investigator is Iracema Leroi, MD, at the University of Manchester, and its aim is to gain a better understanding of interactions between hearing, vision, and cognitive/emotional systems.
Screenings for Cognitive Disorders and Depression
So what does all this mean for today’s dispensing professional? First, I believe the evidence strongly suggests hearing care clinicians should be screening older patients for cognitive disorders, then referring them when appropriate. Some possible screening methods have recently been detailed in articles by Robert Sweetow25 in Audiology Today and Beck et al26 in Hearing Review. Additionally, an overview by Jing Shen and colleagues27 in AJA provides excellent information about cognitive impairment in older adults and available screening tools for use by audiologists.
Likewise, screening for depression and appropriate referral should also be added to any comprehensive audiological evaluation procedure. Although most hearing care professionals are unaware and perhaps uncomfortable about using the available tools for screening depression, there are many methods available. It should be mentioned that depression screening is, in fact, a currently required component in tinnitus evaluation (92625).
Conclusions and Caveats
Contrary to what many in the healthcare community assume, age-related hearing loss (ARHL) is not a benign consequence of aging. ARHL is associated with a number of psychosocial and physiological conditions. However, I can’t emphasize enough that none of the research mentioned here has yet determined causal relationships between hearing loss and other chronic conditions. It would be irresponsible and unethical for dispensing professionals to tell their patients that the science indicates otherwise.
However, what dispensing professionals can do is to be aware of and ask their patients about the above conditions. Hearing care professionals should also keep abreast of the literature concerning chronic conditions associated with hearing loss. Finally, professionals should establish a network of specialists to whom they can refer patients—including primary care physicians, psychologists, gerontologists, and neuropsychologists—especially for those who are in need of more care relative to cognitive function and/or depression.
Acknowledgements
This article summarizes information originally presented in a webinar and white paper by the author, which were sponsored by Hamilton CapTel.
View the 38-minute webinar by Dr Abrams online, courtesy of Hamilton CapTel, by pointing your web-browser to: https://goo.gl/AtTKcr and following the links.
Correspondence to Dr Abrams at: [email protected]
Original citation for this article: Abrams H. Hearing loss and associated comorbidities: What do we know? Hearing Review. 2017;24(12):32-35.
References
-
Agrawal Y, Platz EA, Niparko JK. Prevalence of hearing loss and differences by demographic characteristics among US adults: Data from the National Health and Nutrition Examination Survey, 1999–2004. Arch Intern Med. 2008;168(14):1522–1530.
-
Sung Y-K, Li L, Blake C, Betz J, Lin FR. Association of hearing loss and loneliness in older adults. J Aging Health. 2015;28(6):979-994. Available at: http://journals.sagepub.com/doi/abs/10.1177/0898264315614570
-
Donovan NJ, Okereke OI, Vannini P, et al. Association of higher cortical amyloid burden with loneliness in cognitively normal older adults. JAMA Psychiatry. December 2016; 73(12):1230-1237. Available at: https://jamanetwork.com/journals/jamapsychiatry/article-abstract/2575729
-
Mener DJ, Betz J, Genther DJ, Chen D, Lin FR. Hearing loss and depression in older adults. J Am Geriatr Soc. September 2013. 61(9):1627-1629. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3773611
-
Hsu W-T, Hsu C-C, Wen M-H, et al. Increased risk of depression in patients with acquired sensory hearing loss: A 12-year follow-up study. Medicine (Baltimore). November 2016. 95(44):e5312. Available at: https://insights.ovid.com/crossref?an=00005792-201611010-00076
-
Li C-M, Zhang X, Hoffman HJ, Cotch MF, Themann CL, Wilson MR. Hearing impairment associated with depression in US adults, National Health and Nutrition Examination Survey 2005-2010. JAMA Otolaryngol Head Neck Surg. April 2014 ;140(4):293-302.
-
Lin FR, Ferrucci L. Hearing loss and falls among older adults in the United States. Arch Intern Med. February 2012;172(4):369-371.
-
Friedland DR, Cederberg C, Tarima S. Audiometric pattern as a predictor of cardiovascular status: Development of a model for assessment of risk. Laryngoscope. March 2009;119(3):473–486.
-
Bainbridge KE, Hoffman HJ, Cowie CC. Diabetes and hearing impairment in the United States: Audiometric evidence from the National Health and Nutrition Examination Survey, 1999-2004. Ann Intern Med. July 2008;149(1):1-10.
-
Horikawa C, Kodama S, Tanaka S, et al. Diabetes and risk of hearing impairment in adults: A meta-analysis. J Clin Endocrinol Metab. January 2013;98(1):51-58.
-
Kim M-B, Zhang Y, Chang Y, et al. Diabetes mellitus and the incidence of hearing loss: A cohort study. Int J Epidemiol. April 2017; 46(2): 717-726. Available at: https://academic.oup.com/ije/article-abstract/46/2/717/2452364?redirectedFrom=fulltext
-
Lin FR, Metter EJ, O’Brien RJ, Resnick SM, Zonderman AB, Ferrucci L. Hearing loss and incident dementia. Arch Neurol. February 2011;68(2):214-220. Available at: https://jamanetwork.com/journals/jamaneurology/fullarticle/802291
-
Gurgel RK, Ward PD, Schwartz S, Norton MC, Foster NL, Tschanz JT. Relationship of hearing loss and dementia: A prospective, population-based study. Otol Neurotol. June 2014;35(5):775-781.
-
Fritze T, Teipel S, Óvári A, Kilimann I, Witt G, Doblhammer G. Hearing impairment affects dementia incidence. An analysis based on longitudinal health claims data in Germany. PLoS ONE. July 2016;11(7):e0156876.
-
Wayne RV, Johnsrude IS. A review of causal mechanisms underlying the link between age-related hearing loss and cognitive decline. Age Res Rev. September 2015;23(B):154-166.
-
Fisher D, Li C-M, Chiu MS, et al. Impairments in hearing and vision impact on mortality in older people: The AGES-Reykjavik Study. Age Ageing. January 2014;43(1):69-76.
-
Rumalla K, Karim AM, Hullar TE. The effect of hearing aids on postural stability. Laryngoscope. March 2015; 125(3):720-723. Epub: October 2014. Available at: http://onlinelibrary.wiley.com/doi/10.1002/lary.24974/abstract
-
Boi R, Racca L, Cavallero A, et al. Hearing loss and depressive symptoms in elderly patients. Geriatr Gerontol Int. July 2012;12(3):440-445.
-
Weinstein BE, Sirow LW, Moser S. Relating hearing aid use to social and emotional loneliness in older adults. Am J Audiol. March 2016; 25:54-61.
-
Dawes P, Emsley R, Cruickshanks KJ, et al. Hearing loss and cognition: The role of hearing aids, social isolation, and depression. PLoS One. March 2015; 10(3):e0119616.
-
Acar B, Yurekli MF, Babademez MA, Karabulut H, Karasen RM. Effects of hearing aids on cognitive functions and depressive signs in elderly people. Arch Gerontol Geriatr. May-June 2011;52(3):250-252.
-
Abrams HB, Kihm J. An introduction to MarkeTrak IX: A new baseline for the hearing aid market. Hearing Review. 2015;22(6):16-22. Available at: https://hearingreview.com/2015/05/introduction-marketrak-ix-new-baseline-hearing-aid-market
-
Munro KJ. Reorganization of the adult auditory system: Perceptual and physiological evidence from monaural fitting of hearing aids. Trends Amplif. September 2008; 12(3): 254-271.
-
Lavie L, Banai K, Karni A, Attias J. Hearing aid-induced plasticity in the auditory system of older adults: Evidence from speech perception. J Sp Lang Hear Res. October 2015; 58:1601-1610.
-
Sweetow RW. Screening for cognitive disorders in older adults in the audiology clinic. Audiology Today. July 2015;27(4):38-43.
-
Beck DL, Weinstein BE, Harvey M. Issues in cognitive screenings by audiologists. Hearing Review. 2016;23(2):36-42. Available at: https://hearingreview.com/2016/01/issues-cognitive-screenings-audiologists
-
Shen J, Anderson MC, Arehart KH, Souza PE. Using cognitive screening tests in audiology. Am J Audiol. December 2016;25(4):319-331.