Audiology & Neuroscience | July 2018 Hearing Review
Towards a new validation method to demonstrate the benefit of hearing aid amplification
Objective assessment of amplification benefit in difficult-to-test populations is a challenge for hearing care professionals (HCPs) globally. Hearing aid technology is evolving at a rapid pace, while sophisticated, objective assessment tools lag behind. There is currently a real and substantial need to develop and explore assessment tools that evaluate amplification benefit.
There has been great interest in using speech-evoked cortical auditory evoked potentials (CAEP) as an objective hearing-aid validation measure, because CAEPs allow us to assess the audibility of speech sounds at the highest (cortical) levels. Here is a review of the research, as well as results from a study of first-time hearing aid users who demonstrated that latencies, and amplitudes are distinctive for speech sounds in different frequency ranges and aided responses improve CAEP amplitude and morphology. The findings suggest the clinical value of CAEPs for assessing cortical changes from amplification, as well as using this data in audiology services to demonstrate patient benefit from amplification.
Besides the lack of evaluation tools available to evaluate amplification benefit, the degree of reported benefit a person receives from hearing aid technology varies considerably.3 In a MarkeTrak VIII survey, new hearing aid users reported improved quality of life with hearing aids due to the advent of open-ear hearing aid technologies.4 However, the percentage ratings for overall satisfaction, benefit, and value reported in this survey were closer to 85%, 90%, and 70%, respectively.5 The lower-value reports from the survey pertain to both cost and benefit. Therefore, design and research efforts should focus on continuing to improve technology and features that increase the value (product/price) of hearing aids.
Multiple factors challenge the processing of speech sounds, including the constantly changing incoming speech signal (with respect to frequency, amplitude, and spatial cues) and the influence of digital processing from specific features (eg, compression and noise-reduction algorithms), the integrity of the peripheral and central auditory system, age-related changes in cognition and attention, and more.6
Understanding CAEPs
While the majority of individuals with peripheral sensorineural hearing loss (SNHL) benefit from amplification, the role of the central auditory nervous system (CANS) in determining amplification effectiveness remains largely unexplored. It is widely accepted that the CANS encodes many critical spectral and temporal cues necessary for speech perception.7-9 Speech-evoked cortical auditory-evoked potentials (CAEPs) are measures of neural detection of acoustic cues in the CANS which relate to speech perception and, therefore, offer promise as a tool to evaluate the neural representation of amplified speech.
There are many types of CAEPs, including the P1-N1-P2 complex, acoustic change complex (ACC), mismatch negativity (MMN), and P300 responses (see Martin et al10). Cortical testing by speech-evoked CAEPs is a reasonable solution to determine the neural response of hearing aid wearers to aided auditory signals.
One popular type of clinical electrophysiological test is the Auditory Brainstem Response (ABR). However, the ABR is recorded within 10 msec after stimulus onset, and may be influenced by the processing delay (throughput time) in most digital hearing aids, which is typically 3-10 msec. While using CAEPs, the responses occur around 100 to 300 msec after a signal is presented, making it unlikely that the digital processing delay will affect aided test results.
The P1-N1-P2 complex. The P1-N1-P2 complex is a CAEP response which assesses the capacity of the auditory cortex to detect acoustic changes within speech stimuli11 and provides a functional measure of the benefit provided by personal hearing aids.12-15 The P1-N1-P2 complex is made up of:
- P1: A vertex-positive peak seen approximately 50 msec after stimulus onset;
- N1: A negative peak occurring approximately 100 msec after stimulus presentation, and
- P2: A positive peak occurring approximately 200 msec after stimulus presentation.
These waveforms are thought to represent the synchronous neural activity of structures in the thalamic-cortical segment of the CANS, and can be elicited by clicks, tones, and speech sounds. The recording of cortical event-related potentials (ERP) to speech stimuli provides insight into the early and late cognitive processes which underlie the detection and discrimination of speech in adults with normal hearing and in those with SNHL.16,17 The amplitude of the P1-N1-P2 complex is typically much larger than that of the ABR, providing a greater signal-to-noise ratio (SNR) advantage, especially in noisy subjects.10 Recording the P1-N1-P2 complex is accomplished using specific speech stimuli which are difficult for people with hearing impairment to perceive, allowing testing at the highest level of the auditory system. This results in cortical potentials suitable for studying the amplification effects of hearing aids.
Using the P1-N1-P2 for Insights into the Auditory System
Previous research has shown that age and SNHL influence the latencies and amplitude of CAEPs. Tremblay et al 17 studied P1-N1-P2 responses in three subject groups: young normal-hearing subjects, older normal-hearing subjects, and older subjects with high- frequency hearing loss. Stimuli included a stepwise voice onset time (VOT) in the /ba/ to /pa/ continuum. Each subject was to determine if two stimulus presentations were the same or different.
Results demonstrated that all subject groups had an increase in P1, N1, and P2 latency when the VOT was increased. Older adults with normal hearing showed longer N1 and P2 latencies than younger adults with normal hearing, while older adults with SNHL showed longer N1 and P2 latencies than older adults with normal hearing. N1 responses were prolonged for the older groups (when compared to the younger group) when the stimuli had longer VOT durations. Additionally, P2 was prolonged in both older groups, regardless of condition. Surprisingly, no significant effects of age or VOT condition were found on amplitude of P1 and N1 responses. This study concluded that aging delays synchronous neural firing of cortical neurons to voicing onset and significantly impacts an individual’s ability to discriminate time-varying speech cues.17
Korczak et al16 examined the effects of hearing loss and amplification on cortical evoked potentials in normal-hearing listeners and hearing aid users with SNHL. The speech stimuli /ba/ and /da/ were presented at 65 and 80 dB SPL and cortical potentials were measured at both intensities in active and passive listening conditions.
Results showed that individuals with hearing loss had longer latencies when compared to the normal-hearing group. Individuals with SNHL were tested in aided and unaided conditions, and latencies decreased and amplitudes significantly increased while subjects wore their hearing aids. The amplitude changes from unaided to aided condition were more significant at the lower intensity level. Hearing aids improved detectability of all cortical ERPs, and generally increased amplitudes, decreased latencies, and improved wave morphology. However, there was considerable variability across subjects with SNHL in the amount of response change from unaided to aided conditions. The authors concluded that personal hearing aids in individuals with SNHL significantly impact the timing and strength of the brain processes involved in the detection and discrimination of speech stimuli.16
Use of CAEPs for Objective Hearing Aid Validation
There has been great interest in using CAEPs as an objective hearing aid validation measure.12,15,16-22 As previously noted, CAEPs allow us to assess audibility of speech sounds at the highest (cortical) levels. Researchers agree that aided CAEP responses to speech stimuli can be measured reliably.12,15,16,20-22 However, there is conflicting evidence in the literature regarding the validity of these potentials in new hearing aid users.
Soundfield presentations of stimuli are available using HEARLab, a system for measuring cortical potentials which was originally developed by Frye Electronics and the National Acoustic Laboratories (NAL) in Australia for the purpose of validating hearing aid fittings in infants and adults. The unique aspect of this system (re: commercial auditory evoked potentials’ systems) is the delivery of speech stimuli via a calibrated loudspeaker. Specially designed active electrodes provide resistance to noise effects from electrical interference from capacitive or inductive coupling.23 There are two modules available for cortical testing:
- Cortical Threshold Evaluation (CTE) provides threshold evaluation via tone burst stimuli, and
- Aided Cortical Assessment (ACA) provides aided cortical testing while listeners wear hearing aid technology.
Several recent studies have investigated the validity of CAEPs in amplification using normal-hearing subjects.22,24,25 Even though use of hearing aids in normal-hearing ears increased the SPL of the stimulus in these studies, they did not increase the sensation level of the stimulus. This is because the internal noise of the hearing aids is sometimes audible to people with normal hearing, thereby decreasing the signal-to-noise ratio (SNR) and the sensation level of the stimulus. Billings et al24,25 showed that amplification effects in normal-hearing ears can result in a reduction in amplitude of the CAEP for the aided (versus unaided) stimuli because of the decreased SNR in the aided condition.
Use of CAEPs for evaluation in New Hearing Aid Users
There is currently limited literature on the efficacy of CAEPs in evaluating amplification effects in new hearing aid users. The purpose of the following study was to illustrate the advantages of using this approach clinically.
Our approach using CAEPs was based on two central hypotheses:
- CAEPs can be useful to assess neural processes associated with aided perception of sounds from different frequency ranges (/m/, /g/, /t/) in new hearing aid users, and
- CAEPs can reflect changes in latency and/or amplitude associated with unaided and aided amplification conditions in new hearing aid wearers.
Methods

Table 1. Hearing threshold levels (dB HL); pure-tone averages (PTA) of 500,1000, 2000 Hz; audiometric thresholds; and word recognition scores (WRS) for the subjects.
Subjects. Subjects consisted of 7 adults (3 males, 4 females) between ages 66 and 77 years (mean age: 72 years) with bilateral SNHL. Table 1 shows audiometric thresholds and word recognition scores for the subjects. All subjects were selected from patient files at the University Speech and Hearing Clinic, and signed informed consent forms in accordance with institutional review board (IRB) guidelines at Auburn University.
All participants were new hearing aid users who had normal otoscopic findings, as well as normal immittance results.26 There was no significant otologic or neurologic history for any of the subjects.
As shown in Table 1, pure-tone thresholds were in the mild to moderately severe range and word recognition accuracy scores were above 70% for all subjects. All subjects were binaural hearing aid users.
Each subject was required to score a minimum of 23 on the Mini- Mental State Examination (MMSE) to reasonably assure normal cognitive function. Prior to cortical function testing, ABR testing was conducted using clicks presented at a high level (80 dB nHL) and slow rate (17.7/second) to assure normal brainstem function (characterized by normal absolute latencies for waves I, III, and V) prior to participation in the study.
Stimuli. All CAEPs were measured with the HEARLab recording system using the Aided Cortical Assessment (ACA) software module. According to the developers of the HEARLab system,27 the purpose of the ACA test is to determine if the subject’s hearing aids have been adequately programmed so that a cortical response is present when listening to the speech tokens. The speech tokens (/m/, /g/, /t/) have durations of 30 msec, 30 msec, and 20 msec (respectively) and have dominant power in the frequency bands 500Hz, 1000Hz, and 3000-4000Hz, respectively, corresponding to low, mid, and high frequencies. The interstimulus interval (ISI) used was approximately 1125 msec, and speech stimuli were presented with alternating polarity to reduce the effect of artifact on the CAEP waveforms.
Procedure. Each speech token was presented in the soundfield within a sound-booth at levels of 65 and 75 dB SPL in the aided and unaided conditions via a speaker placed one meter in front of the participant at 0° azimuth. Our test protocol required that each speech token reach 100 accepted epochs before moving on to the next testing condition. Prior to beginning the ACA assessment, the soundfield was calibrated and electrodes were placed on the subject’s forehead (Fpz/ground electrode), inverting mastoid (M1/M2 electrode), and vertex (Cz/non-inverting electrode). The vertex (Cz) location was selected for non-inverting electrode placement because adult CAEPs (P1-N1-P2) are generated closer to the supratemporal cortex.28-31 In a neural source modeling study, adult CAEPs showed a predominant fronto-central or centro-parietal location with maximal activity concentrated closer to the vertex.32
Cortical ERPs. For this study, the cortical responses of interest were the P1, N1, and P2 responses. Each response originates at a different level of the auditory cortex and occurs at a specific point in time (latency) following the onset of the auditory stimulus. The published norms for adults10 indicate mean P1 latencies occur between 40-75 msec, mean N1 latencies occur between 75-150 msec, and mean P2 latencies occur between 150-300 msec.
Hearing aids. All subjects were tested with their own individual hearing aids to study the in-situ benefits of their own instruments during aided testing. To maintain equivalence, technology selected for instruments from two different manufacturers was kept at a similar level and all hearing aids were receiver-in-the-ear (RITE) styles. The RITE (or receiver in the canal, RIC) is extremely popular with HCPs and patients, accounting for the vast majority (69%) of all fittings in 2017.33 An ultra-thin wire is connected to the hearing aid which is coupled to the speaker in the ear canal with a soft, non-occluding ear dome.
All hearing aids were programmed according to manufacturer-supplied first-fit algorithms on the NOAH platform using pure-tone thresholds. Electroacoustic analyses of hearing aids were conducted prior to testing for each subject and real-ear insertion gain measures were completed for each subject. Compression ratios (approximately 2:1) and short attack and longer release times were assured for all hearing aids. Insertion gain measurements were used to determine sensation levels (SLs) for analysis of CAEP amplitudes.
Automatic detection of cortical responses. A statistical algorithm has been developed at NAL for the HEARLab system to provide an automated method of response detection and discrimination. This method does not rely on a template for response detection, but instead uses information from several contributing portions of the response waveform while discounting non-contributing portions of the waveform.23 A Hotelling T2 statistic provides statistical analysis by dividing each response into 50 milliseconds time bins, averaging data points within each bin, using the averages as variables in analysis, and finally, calculating the probability of the response versus random noise. The algorithm enables automated detection of cortical responses and has been validated by Golding et al34 who compared the detection of CAEPs using an automated statistical algorithm with visual detection by a group of expert examiners.
Golding and colleagues looked at the possibility of detection of CAEPs when stimuli were present and also the absence of CAEPs when no stimuli were present in 10 adult listeners. Their study compared the sensitivity and specificity of CAEP response detection using two different methods: visual detection by four expert examiners, and detection by a Hotelling’s T2 statistical algorithm. Results showed that the Hotellings’s T2 algorithm proved to be more sensitive than composite examiner judgments in differentiating CAEPs from random electrical noise activity for sensation levels of 10 dB or greater, equal in sensitivity to the best of individual expert examiners, slightly better in response detection than composite examiners at low sensation levels (10 dB SL) for increased data sizes and reduced variance, and equally sensitive to majority of examiners when stimuli were presented at threshold for small data sets.34
Results
Waveform analyses. Waveform components P1-N1-P2 were evaluated in terms of latency and amplitude measures under unaided and aided conditions. P1 was defined as the largest positive peak following stimulus onset between 40-150 msec; N1 was defined as the largest negative deflection following P1 between 75-200 msec; and P2 as the following positive peak between N1 and 300 msec. Amplitudes were measured from baseline to component peak.
The effects of factors were studied by looking at amplification (unaided versus aided) and soundfield stimulus level (65 dB versus 75 dB) on P1-N1 amplitudes. Repeated measures of Analyses of Variance (ANOVA) indicated statistically significant effects of amplification (Table 2). There were no significant (p>0.05) differences between level (65 versus 75 dB) effects on P1-N1 amplitude.
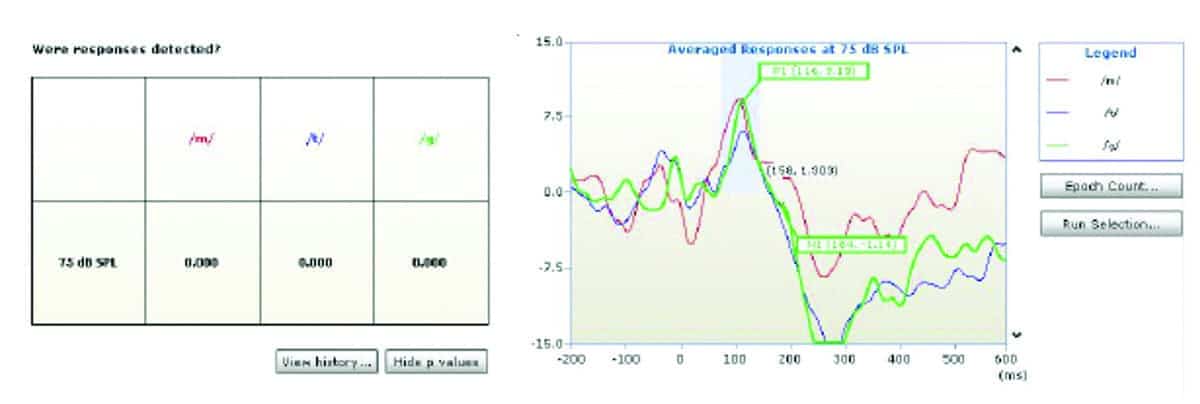
Figure 1 shows an example of a P1-N1-P2 response obtained under unaided conditions and Figures 2-4 show aided responses to /m/, /g/, /t/ stimuli for the same subject. The amplitudes clearly increased from unaided to aided condition. The morphology of responses improved and latencies were clearly identified in the aided conditions in Figures 2, 3, and 4. As explained above, the HEARLab system allows automatic detection of CAEP responses using a statistical algorithm and these are shown in the patient responses in Figures 1-4. The probabilities of response changed from statistically insignificant (p>0.05) in the aided condition (Figure 1) to significant detection probabilities in the aided (p<0.05) conditions for /m/, /g/, /t/ respectively in Figures 1-4.
Discussion
1) Effects of speech stimuli on CAEPs. Speech stimuli from low-frequency (/m/), mid-frequency (/g/), and high-frequency (/t/) regions were used for testing. As shown in the aided conditions in Figures 2-4, cortical responses to speech sounds from low- (/m/), mid- (/g/), and high- (/t/) frequency regions appear to evoke distinct neural patterns. Our results lend support to previous studies by Tremblay, Piskosz, and Souza17 who showed that speech stimuli /si/ and /?i/ stimuli produced different neural responses in individuals with normal hearing. They also reported that N1 responses to /s/ were much larger in amplitude than response to /?/).
Tremblay et al22 studied normal-hearing subjects to allow separation of the effects of hearing loss and amplification. The purpose of their study was to determine whether 20 dB of hearing aid gain would alter cortical potential waveforms obtained for /si/ and /?i/ speech stimuli (a challenging task for individuals with high-frequency sloping hearing loss) presented at 64 dB peak-to-peak equivalent (ppe) SPL. Although Tremblay et al22 found that distinct neural patterns elicited by different speech stimuli were evident in aided conditions, there were no significant differences between aided and unaided cortical responses in response to either speech stimulus. Specifically, adding 20 dB of gain resulted in no significant increase in N1 amplitude or decrease in N1 latency.
2) Effects of amplification on CAEPs. As shown in Figures 1-4, CAEP responses to speech sounds improved in amplitude and morphology from unaided to aided conditions. Aided responses clearly indicate a greater signal reaching the brain—reflected by greater CAEP amplitudes for speech sounds.
Research on the use of cortical potentials in amplification has seen a resurgence following a study by Korczak et al16 who compared the cortical responses of subjects with SNHL to those of normal-hearing subjects by examining cortical potentials to speech stimuli /ba/ and /da/. Overall, they found that for aided cortical responses, amplitudes were 50% larger and latencies were 30 ms shorter than for unaided responses when stimuli were presented at the same input level.
Previous studies of cortical potentials under unaided and aided conditions have been conducted in listeners with normal hearing and yielded different results. Billings et al19 completed a follow-up study in normal-hearing subjects by using 1000 Hz tonal stimuli to compare aided and unaided cortical potentials. The digital hearing aid used in that study was omnidirectional and provided 20 dB of gain from 250-5000 Hz. Similar to the Tremblay et al (2006)22 findings, adding 20 dB of hearing aid gain did not result in an amplitude increase or latency decrease compared with unaided cortical responses at the same input level.
Billings et al25 investigated the effects of SNRs on unaided and aided CAEPs in nine normal-hearing adults. Four levels of gain (0, 10, 20, and 30 dB SPL) were used, and the authors speculated that hearing aid gain would introduce circuit noise and amplify ambient noise in the aided condition, thereby reducing the SNR in the aided condition. In our study, we were unable to obtain actual SNRs for our subjects, but we were able to determine sensations levels (SLs) for unaided and aided conditions.
Cortical potentials can be of particular value for infants and children who are unable to provide subjective measures of aided benefit. Researchers at the NAL have also conducted several aided cortical potential studies on infants with moderate to profound hearing loss.20,21,35 Purdy et al21 outlined a study on aided cortical potentials in infants with SNHL and reported aided cortical potentials were consistently present in infants with moderate SNHL but were only present in half of the cases with profound hearing loss.
In a separate and recent study, soundfield CAEPs for speech sounds (/m/, /g/, /t/) were obtained on a HEARLab system from 24 normal-hearing adults with simulated conductive loss (via earplugs). The automated Hotelling T2 statistical algorithm found good response detection at all levels except for the lowest presentation levels, indicating the validity of the HEARLab system for adults with simulated hearing loss.
Our study results further indicate that soundfield measures of N1-P2 amplitude at varying sensation levels can provide a tool to reflect amplification effects in elderly adults.
3) CAEP utility to evaluate plasticity changes from amplification. Increases in N1-P2 amplitudes have been shown to reflect plasticity following auditory training in adults and may reflect possible increases in neural synchrony.36-37 Previous studies have also shown that increasing N1-P2 amplitudes in developing children and adults may reflect an increased refractory period with maturation.38 It is plausible that amplification effects provide similar changes in neural synchrony for elderly listeners and these effects are measurable using cortical amplitudes.
CAEPs as a Clinical Tool for Verifying Hearing Aid Fittings
This study investigated the effects of digital amplification technology with regard to amplitudes and latencies of CAEPs generated by speech sounds from different frequency ranges (/m/, /g/, /t/) in new adult hearing aid users with SNHL and changes in cortical processing of speech sounds /m/, /g/, /t/ from unaided to aided amplification conditions. Results showed latencies and amplitudes were distinctive for speech sounds /m/, /g/, and /t/ and aided responses improved in amplitude and morphology.
Clinicians and researchers in audiology may wish to record CAEPs under unaided and aided conditions to verify, demonstrate, and confirm physiological changes associated with hearing aid amplification, particularly in difficult-to-assess situations.
Acknowledgments
Portions of this study were presented at the 2011 meeting of the American Auditory Society (AAS) in Scottsdale, Ariz. The author thanks Douglas L. Beck, AuD, senior editor of clinical research at Hearing Review and executive director of academic sciences at Oticon Inc for his review and editorial comments used in the preparation of this manuscript. Finally, thanks to George and the late Sallie Frye at Frye Electronics Inc for providing the equipment to conduct the study.
References
-
Benson D, Clark TM, Johnson JS. Patient experiences with multiband full dynamic range compression. Ear Hear. 1992;13(5):320-330.
-
Boothroyd A, Springer N, Smith L, Schulman J. Amplitude compression and profound hearing loss. J Sp Hear Res. 1988;31:362-376.
-
Yund EW, Buckles KM. Multichannel compression hearing aids: Effect of number of channels on speech discrimination in noise. J Acoust Soc Am. 1995;97(2):1206-1223.
-
Kochkin S. MarkeTrak VIII. Patients report improved quality of life with hearing aid usage. Hear Jour. 2011;64(6):25-32.
-
Kochkin S. Marketrak VIII. Mini BTEs tap new market, users more satisfied. Hear Jour. 2011;64(3):17-24.
-
Souza PE, Tremblay KL. New perspectives on assessing amplification effects. Trends Amplif. 2006;10(3):119-143.
-
Eggermont JJ. Representation of a voice onset time continuum in primary auditory cortex of the cat. J Acoust Soc Am. 1995;98(2 Part 1):911-920.
-
Eggermont JJ. Neural correlates of gap detection in three auditory cortical fields in the cat. J Neurophysiol. 1999;81(5):2570-2581.
-
Steinschneider M, Volkov IO, Noh MD, Garell PC, Howard MA III. Temporal encoding of the voice onset time phonetic parameter by field potentials recorded directly from human auditory cortex. J Neurophysiol. 1999;82(5):2346-2357.
-
Martin BA, Tremblay KL, Korczak P. Speech evoked potentials: From the laboratory to the clinic. Ear Hear. 2008;29(3):285-313.
-
Martin BA. Cortical, auditory, evoked potentials in response to changes of spectrum and amplitude. J Acoust Soc Am. 2000;107(4):2155.
-
Gravel J, Kurtzberg D., Stapells D., Vaughan H, Wallace I. Case Studies. Sem Hear. 1989;10:272-287.
-
Kurtzberg D. Cortical event-related potential assessment of auditory system function. Sem Hear. 1989;10:252-261.
-
Oates PA, Kurtzberg D, Stapells DR. Effects of sensorineural hearing loss on cortical event-related potential and behavioral measures of speech-sound processing. Ear Hear. 2002;23(5):399-415.
-
Rapin I, Graziani LJ. Auditory-evoked responses in normal, brain-damaged, and deaf infants. Neurol. 1967;17(9):881.
-
Korczak P, Kurtzberg D, Stapells DR. Effects of sensorineural hearing loss and personal hearing aids on cortical event-related potential and behavioral measures of speech-sound processing. Ear Hear. 2005;26(2):165-185.
-
Tremblay KL, Piskosz M, Souza P. Effects of age and age-related hearing loss on the neural representation of speech cues. Clin Neurophysiol. 2003;114(7):1332-1343.
-
Dillon H. So, baby, how does it sound? Cortical assessment of infants with hearing aids. Hear Jour. 2005;58(10):10-17.
-
Billings CJ, Tremblay KL, Souza PE, Binns MA. Effects of hearing aid amplification and stimulus intensity on cortical auditory evoked potentials. Audiol Neurotol. 2007;12:234-246.
-
Golding M, Pearce W, Seymour J, Cooper A, Ching T, Dillon H. The relationship between obligatory cortical auditory evoked potentials (CAEPs) and functional measures in young infants. J Am Acad Audiol. 2007;18(2):117-125.
-
Purdy SC, Katsch R, Dillon H, Storey L, Sharma M, Agung K. Aided cortical auditory evoked potentials for hearing instrument evaluation in infants. A Sound Foundation Through Early Amplification. 2001;5(2):115-127.
-
Tremblay K, Billings CJ, Friesen LM, Souza PE. Neural representation of amplified speech sounds. Ear Hear. 2006;27(2):93-103.
-
Dillon H, Van Dun B, Carter L, Gardner-Berry K. Evaluating speech detection in aided infants and estimating pure tone thresholds in unresponsive adults: Auditory-evoked cortical potentials. Paper presented at: AudiologyNOW! 2010; April 14-17, 2010; San Diego, CA. http://slideplayer.com/slide/4825489/
-
Billings CJ, Tremblay KL, Stecker GC, Tolin WM. Human evoked cortical activity to signal-to-noise ratio and absolute signal level. Hear Res. 2009;254(1-2):15-24.
-
Billings CJ, Tremblay KL, Miller CW. Aided cortical auditory evoked potentials in response to changes in hearing aid gain. Int J Audiol. 2011;50(7):459-467.
-
Roup CM, Wiley TL, Safady SH, Stoppenbach, DT. Tympanometric screening norms for adults. Am J Audiol. 1998;7:55-60.
-
National Acoustics Laboratory (NAL). HEARLab System Manual. 2010. Sydney, Australia: NAL. Available at: https://hearlab.nal.gov.au/wp-content/uploads/sites/3/2017/10/HEARLab-Manual.pdf.
-
Hari R, Kaila K, Katila T, Tuomisto T, Varpula T. Interstimulus interval dependence of the auditory vertex response and its magnetic counterpart: Implications for their neural generation. Electroencephalography Clin Neurophysiol. 1982;54(5):561-569.
-
Lu T, Liang L, Wang X. Neural representations of temporally asymmetric stimuli in the auditory cortex of awake primates. J Neurophysiol. 2001;85(6):2364–2380.
-
Picton TW, Alain C, Woods DL, et al. Intracerebral sources of human auditory-evoked potentials. Audiol Neurotol. 1999;4:64-79.
-
Scherg M, von Cramon D. Evoked dipole source potentials of the human auditory cortex. Electroencephalography Clin Neurophysiol. 1986;65(5):344-360.
-
?eponien R, Rinne T, Näätänen R. Maturation of cortical sound processing as indexed by event-related potentials. Clin Neurophysiol. 2002;113(6):870-882.
-
Strom KE. Hearing aid sales increase by 3.4% in 2017. Hearing Review. 2018;25(2):6.
-
Golding M, Dillon H, Seymour J, Carter L. The detection of adult cortical auditory evoked potentials (CAEPs) using an automated statistic and visual detection. Int J Audiol. 2009;48(12):833-842.
-
Pearce W, Golding M, Dillon H. Cortical auditory evoked potentials in the assessment of auditory neuropathy: Two case studies. J Am Acad Audiol. 2007;18(5):380-390.
-
Tremblay KL, Piskosz M, Souza P. Aging alters the neural representation of speech cues. NeuroReport. 2002;13(15):1865-1870.
-
Tremblay K, Kraus N, McGee T, Ponton C, Otis B. Central auditory plasticity: Changes in the N1-P2 complex after speech-sound training. Ear Hear. 2001;22(2):79-90.
-
Gilley PM, Sharma A, Dorman M, Martin K. Developmental changes in refractoriness of the cortical auditory evoked potential. Clin Neurophysiol. 2005;116(3):648-657.


CORRESPONDENCE can be addressed to Dr Krishnamurti at: [email protected]
Citation for this article: Krishnamurti S, Wise L. Effects of amplification on cortical electrophysiological function. Hearing Review. 2018;26(7):26-32.
MORE ON “AUDIOLOGY & NEUROSCIENCE” from this special July 2018 edition of The Hearing Review:
Clinical Speech Audiometry in the Age of the AERP, by James Jerger, PhD.
Cortical Neuroplasticity in Hearing Loss: Why It Matters in Clinical Decision-Making for Children and Adults, by Anu Sharma, PhD, and Hannah Glick, AuD.
Effects of Amplification on Cortical Electrophysiological Function, by Sridhar Krishnamurti, PhD, and Larry Wise, AuD.
Cochlear implants: Considerations Regarding the Relationship between Cognitive Load Management and Outcome, by Edward Overstreet, PhD, and Michel Hoen, PhD.
Dementia Screening: A Role for Audiologists, by Douglas L. Beck, AuD, Barbara R. Weinstein, PhD, and Michael Harvey, PhD, ABPP.