When should cochlear implantation be performed for ANSD patients?
This case study shows why, if cochlear implantation is being considered for a child with ANSD, it might be necessary to delay the procedure until the child is slightly older than the 12- to 18-month age range. This will allow time to gain more details about hearing sensitivity and auditory skills development, as well as to permit the multidisciplinary team and family members adequate time to determine the benefit of appropriately fit amplification.
Auditory neuropathy spectrum disorder (ANSD) is the recently adopted term for an auditory disorder that is characterized by apparently normal outer hair cell function in combination with impaired or absent conduction of synchronous signals by the auditory nerve. When the disorder was first identified, described, and labeled as auditory neuropathy, it was postulated that it might be neural in origin.1,2 However, it has since been determined that ANSD encompasses a heterogenous population with multiple possible sites of lesion—despite the fact that a similar constellation of test findings are present across patients.
Consequently, a number of therapeutic interventions have been suggested or recommended, and no single intervention appears to be best in all cases of ANSD. Multiple studies support the benefits of cochlear implantation for individuals with this disorder.3,4 There are, however, instances in which cochlear implantation is not appropriate or may have much more limited outcomes.5-7
Use of amplification as part of the intervention process in ANSD has been controversial, with some authors suggesting that amplification devices are generally of little or no benefit.8,9 Other studies report positive outcomes with the use of amplification in some cases and recommend consideration of a trial with hearing aids in most cases of patients newly diagnosed with ANSD when hearing sensitivity is not in the normal range.4,10
The following case study describes the diagnosis of ANSD in a child and illustrates use of amplification as well as cochlear implantation for remediation in this disorder.
Case Study of Child With ANSD
This case study involves a male first examined in 2006 and currently followed by the Cochlear Implant Team at our center. The child is a twin who was delivered 3 months prematurely and spent 3 months in the neonatal intensive care unit. Per his parents’ report, he passed newborn hearing screening for one ear.
Initial diagnostic assessment of hearing status was conducted at age 4 months. High frequency tympanometry indicated a flat tympanogram for the left ear, but no reliable acoustic immittance results were obtained for the right ear due to the extremely small size of the ear canal. Distortion product otoacoustic emissions (DPOAE) were absent for both ears. Auditory brainstem response (ABR) testing yielded no synchronous neural responses for click stimuli or for tone burst stimuli at 250 Hz and 1000 Hz for the right ear. In the left ear, a threshold was measured at 70 dBnHL for 250 Hz tone bursts, but no responses were elicited for tone bursts at 1000 Hz or for broadband click stimuli.
Using results of the ABR testing, this child was fit with amplification approximately 3 months after identification of the hearing loss. Amplification targets were generated from the Desired Sensation Level (DSL) prescriptive method. Consistent hearing aid use was not established, however, due to feedback issues related to very small ear canals, as well as ear drainage from recurrent middle ear infections. During his first few months of life, he was further diagnosed with retinopathy of prematurity, which was treated surgically. Failure to thrive and delayed motor milestones were also present.
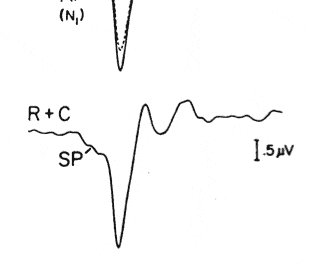
Figure 1. Waveforms recorded at the time of cochlear implant candidacy evaluation. Stimuli were tonebursts at 250, 1000, 2000, and 4000 Hz. Note that there are no repeatable neural responses, but phase-reversing cochlear microphonic responses can be observed in the first few milliseconds of the tracings for 500, 1000, and 2000 Hz. A control trial with the stimulus on and the tubing detached from the insert earphone yielded nothing resembling a neural response or cochlear microphonic.
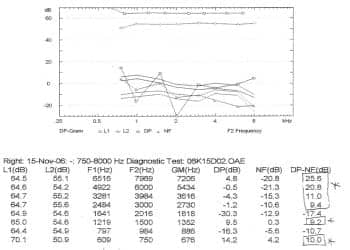
Figure 2. Distortion product otoacoustic emissions (DPOAE) measured at the time of the CI candidacy evaluation. The emissions (open circles) are clearly visible above the noise floor (open triangles) for the frequency range from 3000 through 8000 Hz. DPOAEs were also present at signal-to-noise ratios greater than 6 dB at 750 and 1500 Hz. This is incongruent with the ABR findings that suggested severe-to-profound hearing loss, and allows for the definitive diagnosis of ANSD.
Cochlear Implantation
In light of the severe-to-profound hearing loss indicated in the ABR results, consideration was given to cochlear implantation when the child was approximately 15 months of age. This had been discussed earlier, but because the child reportedly showed some unaided responses to environmental sounds, his parents questioned whether he might have had an improvement in hearing sensitivity and were hesitant to pursue a cochlear implant (CI).
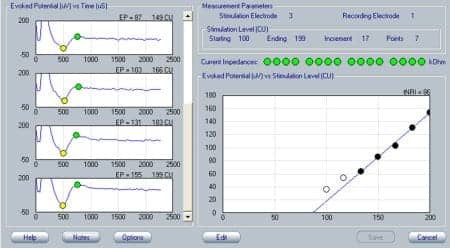
Figure 3. Electrically evoked compound action potentials (ECAP) recorded at the time of initial activation of the cochlear implant. The ECAP is characteristically shown as a negative deflection (N1) followed by a positive peak (P1). Responses for increasing stimulus levels are shown from top to bottom on the left side of the panel. ECAP response amplitudes (vertical axis) are plotted as a function of stimulation level (horizontal axis) on the right side of the panel. The solid circles indicate response amplitudes that are above the recording system’s noise floor.
A CI candidacy evaluation was conducted to determine the best course of action. The child’s developmental delays precluded reliable behavioral audiometry, so ABR testing was repeated. No neural responses were present for either ear, but cochlear microphonics (CM) were observed for tone bursts at 250, 1000, and 2000 Hz, bilaterally (Figure 1). The child had patent tympanostomy tubes at the time of testing. Although tympanostomy tubes can sometimes obscure otoacoustic emissions, DPOAEs were present with amplitudes in the normal range for multiple frequencies in both ears (Figure 2). These findings of outer hair cell function in conjunction with a lack of synchronous response from the auditory nerve resulted in the diagnosis of what at that time was labeled “auditory neuropathy/auditory dys-synchrony” and what is now referred to as ANSD.
Significant speech-language delay was identified through testing done as part of the candidacy evaluation, and the child’s score on the Infant-Toddler Meaningful Auditory Integration Scale (IT-MAIS)11 was 3/40 for a normal-hearing age equivalency of less than 1 month of age, indicating a marked delay in development of auditory skills.12 Intervention choices were impacted by the inability to fit amplification from ABR findings in patients with ANSD, and this child’s developmental delays appeared likely to preclude reliable behavioral audiometry results for some time to come. In light of these factors and because of the body of literature available that supported cochlear implantation as a viable option for remediation in cases of ANSD, the decision was made to proceed with a CI for one ear.
Appeal of an initial insurance denial and continuing issues with ear infection delayed the CI surgery until the child was 22 months of age, when he received an Advanced Bionics HR90K device for the right ear. At the initial activation of the implant, electrically evoked compound action potential (ECAP) measurements revealed synchronous activity from the auditory nerve (Figure 3). A few weeks after activation of the CI, a behavioral audiogram obtained with the device in use showed thresholds in the 30 to 40 dBHL range for narrowband noise stimuli from 250 Hz through 6000 Hz (Figure 4).
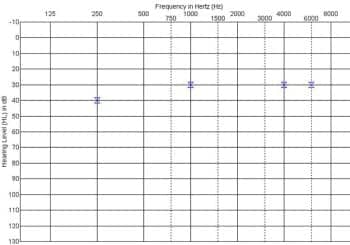
Figure 4. Behavioral thresholds obtained via visual reinforcement audiometry with the cochlear implant in use on the right ear a few weeks after initial activation of the device.
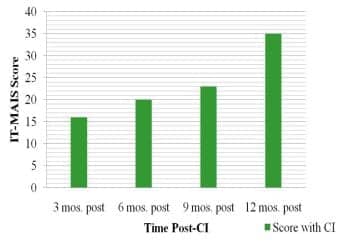
Figure 5. Progress in development of auditory skills as represented by scores on the IT-MAIS obtained at 3-month intervals during the first year following cochlear implantation.
Progress of auditory skills development was tracked using the IT-MAIS (Figure 5). In the first 12 months following activation of the implant, age equivalency (AE) scores showed a steady progression, such that the child’s performance at 1 year post-implantation was comparable to a normal-hearing AE of 14.7 to 17.2 months. This suggested at least 1 year of development in 1 year of time, and indicated that the implant was providing significantly more benefit than had been experienced with hearing aids.
Hearing and Fitting
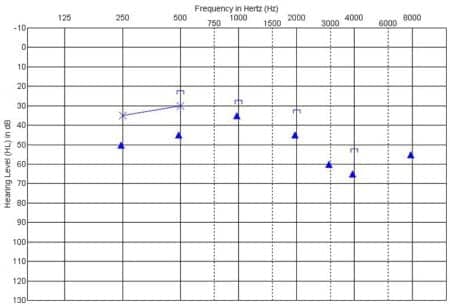
Figure 6. Behavioral audiogram showing unaided thresholds obtained via conditioned play audiometry for the left (non-implant) ear approximately 15 months after receiving the CI in the right ear. The filled triangle symbols indicate thresholds with ER-3A insert earphones, and thresholds with TDH supra-aural earphones are represented by the X symbol. Results indicate a mild to moderately severe sensorineural hearing loss.
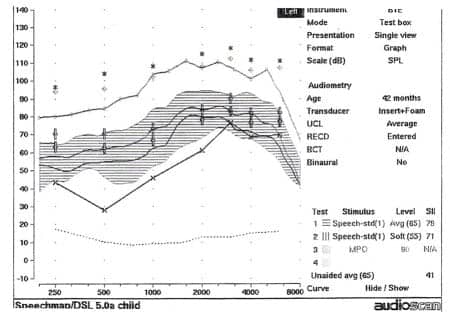
Figure 7. Speech SPL-o-gram from the fitting of a hearing aid on the left ear. Frequency (in Hz) is represented on the horizontal axis, and amplitude (in dB) is represented on the vertical axis. Amplification targets were generated by the DSL prescriptive method. The child’s auditory thresholds, converted to dBSPL, are represented by the X symbols connected by a solid line. The amplified long-term average speech spectrum (LTASS) with an overall input level of 65 dBSPL is shown by the cross-hatched area, and maximum output of the hearing aid is shown by the solid line stretching across the upper portion of the SPL-o-gram. A measured real-ear-to-coupler difference (RECD) was used in conjunction with 2cc coupler measurements to obtain estimates of amplified output in the patient’s ear.
At 15 months post-implantation, the recommendation was made to consider the option of fitting a hearing aid on the child’s non-implant ear. The benefits of bimodal hearing over use of the cochlear implant alone for patients with residual hearing in the ear contralateral is supported in the literature,13-15 and it is common practice in our clinic to discuss it with our patients who have a unilateral cochlear implant. As mentioned previously, some researchers have indicated that one can expect hearing aids to be of little or no benefit for patients with ANSD. However, other reports have indicated that this is not universally the case.10 Based on these latter findings, as well as on some positive clinical experience we have had involving using hearing aids for patients with this disorder, the decision was made to proceed with a new hearing aid trial for this child.
As part of the process to pursue this treatment option, unaided audiometric testing was completed for the unimplanted left ear. Surprisingly, thresholds were obtained in the mild to moderately severe hearing loss range (Figure 6). This degree of residual hearing was well within a range that would be considered potentially responsive to treatment with amplification, so a trial was initiated with a hearing aid on the left ear in addition to the CI on the right.
Amplification was fit using the DSL prescriptive technique. Figure 7 shows the Speech SPL-o-gram from the hearing aid fitting. The device appeared to be providing audibility of the majority of the portion of the conversational level long-term average speech spectrum from 250 Hz to 8000 Hz. It was possible to establish consistent use of the hearing aid with no signs of loudness intolerance or apparent degradation of auditory performance.
Figure 8 shows the child’s current audiogram obtained with the cochlear implant in use. Figure 9 illustrates recently obtained “auditory only” speech perception scores. For the NU-CHIPs closed-set monosyllable test, comparisons can be made across the cochlear implant alone, hearing aid alone, and bimodal (cochlear implant + hearing aid) conditions. As one can see, there is no significant difference between the CI alone and hearing aid alone conditions, and combining the acoustic signal from the hearing aid with the electrical stimulation from the CI does not result in a decrement in performance on this particular test. Also shown in the graph is bimodal performance on Subtest V of the Test of Auditory Comprehension (TAC) condition. This is a closed-set task that assesses discrimination and memory-sequencing abilities for messages containing two critical elements.
A recent speech-language evaluation (SLE) at age 4 years 7 months (55 months) yielded standard scores on the Preschool Language Scale (PLS-4) of 73 for Auditory Comprehension and 61 for Expressive Communication. This equates to age equivalencies of 37 months for receptive skills and 22 months for expressive skills. The speech pathologist reported that this child’s coarticulation remains imprecise, causing problems with intelligibility for connected utterances. Nevertheless, his vowel production was normal and he exhibited a fairly good repertoire of consonant sounds. Overall, although he still exhibits speech-language delays, he was judged to have made good progress in the 18 months since his previous SLE.
Discussion
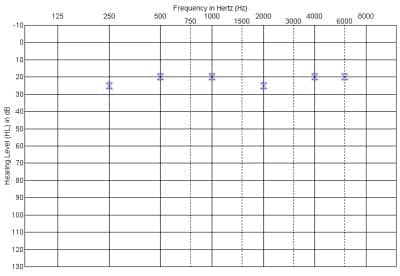
Figure 8. Recent behavioral audiogram obtained with the cochlear implant in use.
A number of key points can be drawn from the child reported here. This case example supports the evidence that has shown that cochlear implantation can be a viable treatment option in some patients with ANSD. It also highlights the question of when cochlear implantation should be performed in a case where behavioral thresholds are not available and the ABR does not serve as a reliable predictor of auditory sensitivity.
Communication outcomes with cochlear implants are correlated to age at implantation, and it is commonly accepted that earlier age of implantation generally yields better outcomes than later age. In fact, the current trend seems to be toward implanting children even before age 12 months, if possible. However, proceeding with implantation before reliable information on auditory sensitivity has been obtained obviously runs the risk of performing the procedure in cases where the hearing loss is less severe than that which is typically considered appropriate for CI candidacy and which may be amenable to treatment with amplification.
There is at least one published report of cochlear implantation with beneficial outcomes in a patient with ANSD and milder degree of hearing loss who did not benefit from amplification.16 However, there are a number of reports detailing individuals diagnosed with ANSD who have aided performance comparable to patients with sensorineural hearing loss of the same degree.3,10
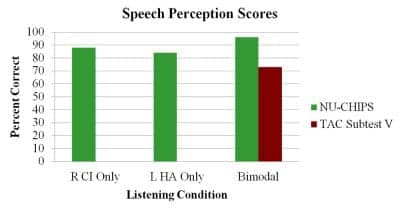
Figure 9. Recently measured speech perception scores obtained for the cochlear implant alone, the hearing aid alone, and bimodal conditions. Speech stimuli were presented in the auditory only mode.
Consequently, cochlear implantation for patients with ANSD and milder hearing loss may be an option, but should not be a foregone conclusion. The limited aided speech perception data gathered for this child would tend to suggest that he is receiving significant benefit from amplification in his non-implant ear. When working with patients with ANSD, it would seem that no intervention option should be automatically discarded on the assumption that it is not beneficial with this population. With regard to young children with ANSD whose hearing sensitivity cannot be determined through behavioral audiometry, it has been suggested that perhaps the best approach for determining appropriate remediation would be to have the child evaluated and followed by a multidisciplinary team (MDT) that is familiar with the characteristics of this disorder and that follows a systematic intervention protocol (see the article by Erin Pinsky, AuD, in this issue of HR).4,17
In retrospect, this case study shows why, if cochlear implantation is being considered for a child with ANSD, it might be necessary to delay the procedure until the child is slightly older than the 12— to 18—month range. This will allow time to gain more details about hearing sensitivity and auditory skills development, as well as to permit the MDT and family members adequate time to determine the benefit of appropriately fit amplification.
ADDITIONAL ONLINE RESOURCE:

“Fitting and evaluating a hearing aid for recipients of a cochlear implant: The NAL approach, by Theresa Ching, PhD, and colleagues.
Acknowledgement
This case study was originally presented at the Starkey First Pediatric Symposium in September 2010.
References
- Sininger YS, Hood LJ, Starr A, Berlin CI, Picton TW. Hearing loss due to auditory neuropathy. Audiology Today. 1995;7:16-18.
- Starr A, Picton TW, Sininger Y, Hood LJ, Berlin CI. Auditory neuropathy. Brain. 1996;119:741-753.
- Rance G, Barker JB. Speech perception in children with auditory neuropathy/dyssynchrony managed with either hearing aids or cochlear implants. Otol Neurotol. 2007;29:179-182.
- Teagle FBT, Roush PA, Woodard JS, Hatch DR, Zdanski CJ, Buss E, Buchman CA. Cochlear implantation in children with auditory neuropathy spectrum disorder. Ear Hear. 2010;31:325-335.
- Buchman C, Roush P, Teagle HFB, Brown CJ, Zdanski C, Grose JH. Auditory neuropathy characteristics in children with cochlear nerve deficiency. Ear Hear. 2006;27:399-408.
- Miyamoto RT, Kirk KE, Renshaw J, Hussain D. Cochlear implantation in auditory neuropathy. Laryngoscope. 1999;109:181-185.
- Walton J, Gibson WPR, Sanli H, Prelog K. Predicting cochlear implant outcomes in children with auditory neuropathy. Otol Neurotol. 2008;29:302-309.
- Berlin CI, Hood L, Morlet T, Rose K, Brashears S. Auditory neuropathy/dys-synchrony: diagnosis and management. Mental Retardation and Development Disabilities Research Review. 2003;9:225-231.
- Berlin CI, Hood LJ, Morlet T, et al. Multi-site diagnosis and management of 260 patients with auditory neuropathy/dys-synchrony. Int J Audiol. 2010;49:30-43.
- Rance G. Auditory neuropathy/dyssynchrony and its perceptual consequences. Trends Amplif. 2005;9:1-43.
- Zimmerman-Phillips S, Robbins AM, Osberger MJ. Infant-Toddler Meaningful Auditory Integration Scale. Sylmar, Calif: Advanced Bionics Corp; 2001.
- Kishon-Rabin L, Taitelbaum R, Elichai O, Maimon D, Debyiat D, Chazan N. Developmental aspects of the IT-MAIS in normal-hearing babies. Israeli J Speech Hear. 2001;23:12-22.
- Ching TYC, van Wanrooy E, Dillon H. Binaural-bimodal fitting or bilateral implantation for managing severe to profound deafness: a review. Trends Amplif. 2007;11:161-192.
- Ching TYC, Hill M, Dillion H, van Wanrooy E. Fitting and evaluating a hearing aid for recipients of a unilateral cochlear implant: the NAL approach, Part 1. Hearing Review. 2004;11(7):14-22,58. Available at: www.hearingreview.com/issues/articles/2004-07_01.asp. Accessed September 9, 2011.
- Ching TYC, Incerti P, Hill M, Brew J. Fitting and evaluating a hearing aid for recipients of a unilateral cochlear implant: the NAL approach, Part 2. Bimodal hearing should be standard for most cochlear implant users. Hearing Review. 2004;11(8):32-40,63. Available at: www.hearingreview.com/issues/articles/2004-08_03.asp. Accessed September 9, 2011.
- Shallop J. Auditory neuropathy/dys-synchrony in adults and children. Seminars in Hearing. 2002;22:215-223.
- Simmons J. Cochlear implants in auditory neuropathy spectrum disorder. Available at: www.asha.org/aud/articles/CochlearImplantsANSD.htm. Accessed March 2011.
Correspondence can be addressed to HR or Jeffrey Simmons, MA, at .
Citation for this article:
Simmons J. Bimodal Stimulation in Auditory Neuropathy Spectrum Disorder: A Case Study Hearing Review. 2011;18(11): 34-38.