ZPower, a developer of rechargeable, silver-zinc miniature batteries, and the manufacturer of the ZPower Rechargeable System for Hearing Aids, announced that as of October 13, 2015 its facility and devices are registered with the FDA. Specifically, ZPower’s facility in Camarillo, California is now an FDA registered Medical Device Establishment for the manufacturing of medical device accessories.
“We’re seeing a growing demand for use of our high energy batteries and electronics in hearing and medical devices, so registration with the FDA helps assure our customers that ZPower meets or exceeds regulatory requirements,” said Ross Dueber, president and CEO of ZPower.
ZPower’s Rechargeable System for Hearing Aids is designed to make it easy to convert many new and existing hearing aids to rechargeable technology. According to a recent MarkeTrak 9 study, rechargeable batteries and rechargeable hearing aids are among the top features sought by hearing aid users.
Disposable hearing aid batteries, which are used by people with varying levels of dexterity, often run out of power in the middle of the day, and require frequent changing. ZPower reports that its rechargeable system offers a full day of continuous power, charges overnight in the hearing aids, takes the place of an estimated 200 disposable batteries, and lasts a full year. The ZPower hearing aid battery is replaced once per year by a hearing care professional so the patient never has to touch a hearing aid battery again.
Source: ZPower


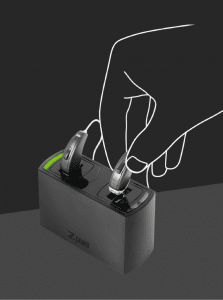




This looks like a really great idea (if it works), although like Debbra, my first concern was that the daily need for charging will make using our recommended daily drying problematic. The zPower system model I’ve seen looks pretty small, which is encouraging. Could the whole system be put into a container for drying while charging? (ie, tupperware with some sort of dri-brick/desiccant?) Or would that interfere with charging? A zPower dryer would be ideal!
I would like to see a rechargeable system that takes in account moisture and wax issues as well because if patients have these issues and do not use some sort of a drying system they have constant problems with recievers burning out and that costs a lot more to all involved versus battery purchases.
Hi Debbra,
Thanks for highlighting these issues. Hearing Review recently reported on a product called “DryDome” that addresses moisture and wax issues in hearing instruments, though the product does not integrate drying with battery recharging (great idea!). Here is a link to the HR article on the new DryDome (https://hearingreview.com/2015/12/eartech-launches-moisture-control-device-hearing-instruments/), in case readers find it helpful.