July 2005 marked the 25th anniversary of the first pediatric cochlear implant in the United States performed by Dr. William House. It was a significant event in hearing care history that created both fanfare and controversy for the House Ear Institute (HEI), which in July 1980 received FDA approval for a clinical trial to implant three patients under the age of 18 with a single-channel cochlear implant. Since that time, cochlear implant (CI) technology has proven to be one of the most significant advances in the treatment of those with profound hearing loss.

But the road wasn’t exactly a smooth one. “There’s a saying in the Old West that it’s the pioneers who get the arrows,” said House, summing up this period during a speech in which he accepted the American Auditory Society’s Lifetime Achievement Award.1 House started his career as a dentist then attended medical school, eventually joining the medical practice of his brother, Howard House, MD. He went on to develop the surgical procedure to gain access to the cochlea via the facial recess from the mastoid to the middle ear. However, before he pioneered this important procedure, a patient in 1957 (House’s second year of practice) brought an article to him about two French surgeons (Dijourno & Eyries2) who had inserted an electrode onto the auditory nerve of a deaf man and discovered that the man could perceive sounds when the nerve was stimulated with current.3
Thus began House’s interest in cochlear implants. By 1961, he had implanted the first two American patients for short-term stimulation of hearing in clinical trials at HEI; however, the lack of biocompatible materials were a large obstacle in those days. Four years later, Dr. House teamed up with engineer Jack Urban to develop a CI system for longterm use. Beginning in the mid-1960s, Blair Simmons at Stanford University, Robin Michelson at UCSF, Graeme Clark at the University of Melbourne, Dr. Claude-Henri Chouard in France, and others began to perform animal and human research to investigate the feasibility of single and multichannel cochlear implants.
In 1969, three more patients at HEI were implanted with a multiple-electrode system, allowing direct electrical connections across the skin,4 and the first “take-home” cochlear implant was developed in 1972. It consisted of a wearable speech processor that interfaced with the House/3M single-electrode implant.
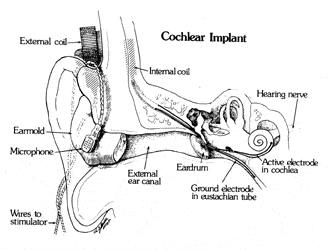
Diagram of anatomical placement of the single channel cochlear implant developed at HEI. Courtesy of HEI.
Other landmarks in cochlear implant research and the commercial production of CIs followed from 1970-1982. It was during this period that HEI, in partnership with 3M Company, implanted five patients through an organized clinical trial. Important research was also conducted at the University of Utah, University of Oregon, and University of Toronto on the viability of a cochlear prosthesis.
In initiating the clinical trials, House and his colleagues often faced open hostility from scientists, particularly neurologists, who believed they were moving too far, too fast. The Deaf Culture movement also objected to the device on numerous grounds. One criticism by scientists to this early work was that a single-electrode device would produce a “Morse-code-like” buzzing, providing only limited contact with the external environment.3 Other critics thought that animal work was needed first to solve potential problems and ensure that continuous electrical stimulation to the cochlea wouldn’t destroy the neural tissue. Essentially, House and most others working on cochlear implant technology felt that it was impossible for animals to, as House put it, “assist with solving many of the pivotal research problems for a system which is intended to provide access to speech.”3 Additionally, critics voiced worries about meningitis caused by infection (eg, otitis media) spreading via the electrode to the cochlea and then to the spinal fluid. However, this had not been observed in stapedectomy patients who had a wire running from the middle ear to the oval window. There also was (and, to some extent, continues to be) debate about single-vs-multiple electrode devices and issues related to tonotopic theory (for an interesting perspective of this topic and an argument for shorter electrodes, see the online article by House3).

Graeme Clark and colleagues at the University of Melbourne published a paper in 1977 that described their cochlear implant—a multichannel device that would eventually become the most prevalent implant, Cochlear Corp’s Nucleus device. That same year, a report by Bilger et al5 looked at 13 adults (11 from HEI’s program and two from Michelson’s UCSF program) who used single-electrode CIs and, in general, the “Bilger Paper” offered positive views about CIs. By 1978, the Univ of Utah had implanted patients with a multichannel device.
House’s initial team included Urban, with the help of Simmons and Michelson. House also signed on a list of medical co-investigator leaders, many of whom went on to have brilliant careers. These included, but are not limited to, Brad Edgerton, Simon Parisier, Ed Maddox, Karen Berliner, Charles Luetje, C.T. Campos, Daniel Bode, David Austin, H.A. Ted Bailey, Jr, Jack Clemis, Charles Phillip Daspit, Bruce Gantz, Michael Glasscock, J.V. David Hough, Sam Kinney, Roger Lindeman, Charles Luetje, H. Edward Maddox, Charles Mangham, Brian McCabe, Tom McMurry, William Meyerhoff, Robert Mischke, Richard Miyamoto, Dennis Pappas, James Pappas, Fred Shaia, James B. Snow, and Richard Wiet.4 Others conducting studies during this same period included researchers like John Frederickson, John Niparko, Mary Jo Osberger, Margaret Skinner, Steven Staller, and Emily Tobey, to name only a few.
Implants Approved for Children
The first pediatric implantation in the US took place in July 1980.5,6 By mid-1981, 178 patients (nine under the age of 18) had received the House/3M single-electrode device: 91 patients through HEI and 87 through the co-investigator sites. House et al reported at that time that only 16 implanted patients had chosen not to use the device on a regular basis.4


The House/3M CI was approved for use in adults by the FDA in 1984. One year later, Cochlear Corp’s Nucleus implant was similarly approved by the FDA. The early 80s also saw the Research Triangle Institute beginning its work on a standard speech processor. This culminated in the introduction of the CIS speech processor, a significant step for improving speech recognition by CI wearers, and non-hardware innovations have been instrumental in subsequent improvements in CI technology.
The primary developments in the late-80s and early-90s seemed to be dominated by multiple-channel devices that were reported to enhance spectral perception and open-set speech understanding. By 1990, the work had advanced to the point where the FDA approved the implantation of children with a multichannel cochlear implant as young as age 2.
It was also during the 1990s that cochlear implants began to make exceptional gains in terms of speech processing, the number of implants being developed, and the number of implants being performed. In 1991, Advanced Bionics Corp introduced the Clarion cochlear implant, and the FDA approved this device for adults in 1997 and for children two years later. Other implants that are currently awaiting FDA approval for the US market include the Med-El and Allhear devices.
To provide some perspective on how fast CIs are progressing, consider this: in 1997 almost 20,000 people had cochlear implants worldwide; 8 years later the number pediatric implant patients is close to 50,000 worldwide, according to HEI. In 30 years, cochlear implants have evolved from highly controversial experimental devices to playing a crucial role in the treatment options of both adults and children with profound hearing loss. w
Acknowledgements
The editor thanks Christa Spieth Nuber at HEI for use of the photos and for providing some of the information in this article. For more about the history of cochlear implants, see House & Berliner,8 Schindler & Merzenich,9 and Koch.10
. Laurie S. Eisenberg, PhD, can be contacted at: [email protected].
References
1. AAS scientific meeting shares research from all disciplines. The Hearing Review. 2000;7(6):66-69.
2. Digjourno A, Eyries C. Prosthese auditve par excitation electrique a distance du nerf sensoriel a P’adie d’un bobinage inclus a demeure. Press Med. 1957;35:14-17.
3. House WF (ed. House D). Cochlear Implants: My Perspective. Aurora, Ore: AllHear Inc. Found at: www.allhear.com/monographs/m-95-htm.html; 1995.
4. House WF, Bode DL, Berliner KI. The cochlear implant: Performance of deaf patients. Hear Instrum. 1981;32(9):13-18.
5. Bilger RC, Black FO, Hopkinson NT, Myers EN, Stenson NR, Vaga A, Wolf RV. Evaluation of subjects presently fitted with implanted auditory prostheses. Ann Otol Rhinol Laryngol. 1977;[Suppl] 86(May-June).
6. Eisenberg LS, House WF. Initial experience with the cochlear implant in children. Annals Otol Rhinol Laryngol. 1982;[Suppl] 91: 67-73.
7. Eisenberg LS, Berliner KI, Thielemeir MA, Kirk KI, Tiber N. Cochlear implants in children. Ear Hear. 1983;4:41-50.
8. House W, Berliner K. Cochlear implants: From idea to clinical practice. In: Cooper H, ed. Cochlear Implants: A Practical Guide. London: Whurr Publishers; 1991: Chapter 2.
9. Schindler RA, Merzenich MM. Cochlear Implants. New York: Raven Press; 1985.
10. Burton Koch D. Cochlear implants: An overview. Found at: www.audiologyonline.com/articles/ arc_disp.asp?id=222; September 2000.