Harnessing technology to expand the reach of hearing healthcare
The rapid growth in global connectivity is opening up access possibilities for delivering hearing healthcare using telehealth models. Capitalizing on these developments requires rethinking current audiological practices and being innovative with the technology at our disposal.
Pure-tone air and bone conduction audiometry has remained the gold standard for clinical diagnosis of hearing loss. It provides the necessary information to characterize the type, degree, configuration, and symmetry of hearing loss directing the necessary audiological or medical intervention required. Despite its importance and straightforward application, the majority of people with hearing loss are unable to access this service.1
Recent estimates indicate a global prevalence for significant permanent bilateral hearing loss in adults of 538 million,2 making hearing loss the most prevalent global disability. More than 80% of these people live in developing countries where hearing healthcare services are absent or very difficult to access.1,3,4 The shortage of hearing healthcare is largely due to the dearth of hearing care professionals, especially audiologists.3,4 In sub-Saharan Africa, for example, many countries have no audiology or ENT services. Of the 46 countries constituting this world region—which comprises almost 1 billion people—there is only one (South Africa) with tertiary-level education for audiologists.
A shortage of hearing healthcare services is not only a problem for the developing world. According to estimates, there is a major capacity shortage in the United States relative to the need for audiograms and the capacity of the current audiological workforce to deliver these.5 Underserved areas also persist in developed countries like Canada and Australia due to traveling distances, rural communities, and geographical and weather obstacles. Utilizing telehealth approaches in hearing healthcare has been suggested as a means of addressing the shortage of appropriate services including pure-tone audiometry.6,7
Providing pure-tone audiometry within a telehealth service-delivery model may bridge the barrier, whatever this may be (eg, geographical, weather, etc), between patients and professionals using information and communication technology. Saving travel costs and time for patients and professionals could be realized, along with optimizing the use of limited resources. This may ensure better service distribution between rural, urban, and inner-city populations irrespective of the geographical spread of hearing care professionals.
Challenges in Tele-audiology

Figure 1. KUDUwave 5000 audiometer (GeoAxon, South Africa) Type 2 clinical audiometer. Hardware encapsulated in the earcups with USB power from notebook and USB response button. Insert earphones covered by circumaural earcups for additional passive attenuation and microphones on the outside of each earcup for active monitoring of environmental noise levels during testing.
Apart from the requirements for sufficient connectivity, there are many challenges related to tele-audiometry that include the equipment, test environments, and considerations related to synchronous and asynchronous telehealth models. Current audiometric equipment is often not suited for telehealth purposes. Remote control during face-to-face tele-audiometry requires computer-operated audiometers. Since the hearing care professional is not on-site during face-to-face tele-audiometry, quality control measures (indications of patient responses, reaction time, etc) become important to ensure valid and reliable test findings. Unfortunately, most audiometry systems at present lack many of these features.
The test environment is also a significant challenge. Diagnostic pure-tone audiometry requires ambient noise levels in the test environment that are sufficiently low and compliant to recommended standards to ensure hearing thresholds can consistently be recorded down to 0 dB HL. This is typically achieved by employing audiometric booths or sound-treated rooms. In most of the underserved areas where teleaudiometry services would be provided, these facilities are not available. Alternative methods, such as increased passive attenuation (eg, combination of insert earphone and circumaural hearing protection) and active noise cancellation, have been proposed.6 Despite such methods, there is still the concern of quality control to ensure valid testing outside a sound booth.
Even when these challenges are overcome, the problem of limited professionals to provide face-to-face tele-audiometry remains. Increasing patient access to real-time audiometry in a synchronous telehealth setup does not increase the capacity of the hearing care professional to provide pure-tone audiograms. Increased patient access to pure-tone audiometry will require utilization of automated audiometry within an asynchronous telehealth model. In such a system, a non-specialist telehealth clinic facilitator may mediate between the patient and on-site equipment (ie, a telehealth-enabled audiometer). Audiograms generated with the automated audiometer are emailed to hearing care professionals for interpretation or uploaded to a centralized server for offline interpretation by them. In this type of setup, only patients for whom these clinicians query the automated audiogram or difficult-to-test patients are reserved for the more time-consuming task of face-to-face tele-audiometry assessments.
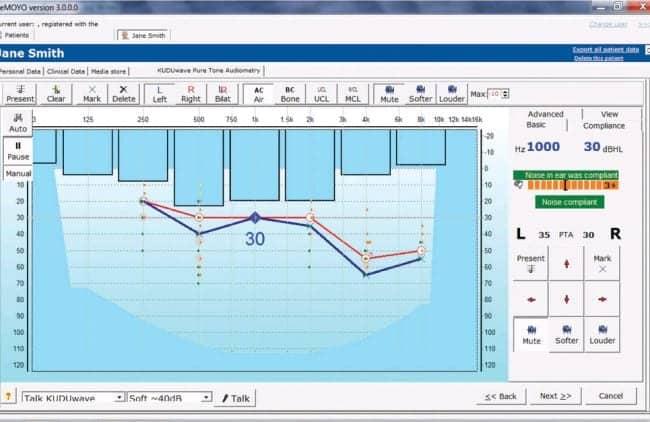
Figure 2. Software-based audiogram constructed during audiometric testing. Active monitoring of noise levels at each octave frequency is illustrated by blue bars (left ear being tested). Green and orange markings on audiogram indicate true positive and negative responses. Yellow markings indicate true negatives, but with noise levels sufficiently high to allow possible masking of a response.
Example of a Tele-audiometry System
A pilot tele-audiology clinic in South Africa is employing a telemedicine-enabled mobile audiometer that incorporates unique quality control features for testing in natural environments, utilizes automated test sequences, and includes synchronous audiometric test features (Figure 1). The KUDUwave audiometer, manufactured by GeoAxon of Pretoria, South Africa, is a Type 2 Clinical Audiometer (IEC 60645-1/2) that is software controlled and operated via a computer notebook. The audiometer hardware is encased in each circumaural earcup and is powered by a USB cable plugged into the notebook.
The transducers include custom insert earphones, which are covered by circumaural cups after insertion, and a B-71 bone oscillator (Kimmetrics, Smithsburg, Md) placed on the forehead with a standard adjustable spring headband held in place on the center of the circumaural headband with a screw fitting (Figure 1). The device has been validated against a standard Type 1 research audiometer for air and bone conduction audiometry.9
Testing outside a sound booth. Testing outside a sound-treated environment is accomplished by the use of passive attenuation provided by the insert earphones covered by the circumaural earcups. These attenuation values exceed those of typical transportable sound-treated booths.9,10 Furthermore the use of two microphones on the outside of each circumaural earcup monitors the environmental noise across octave bands during testing and is visually represented on the software (Figure 2). Noise monitoring is based on the maximum permissible ambient noise levels according to current standards, taking into account the attenuation provided by the insert earphones and circumaural earcups.

Figure 3. Patient being tested with the KUDUwave audiometer at a pilot hearing telehealth clinic in South Africa. He is holding a response button connected to the audiometer. The notebook computer uploads all test information to a secure server using a 3G cellular Internet connection for asynchronous interpretation.
This allows for testing in natural environments while receiving information on the compliance of ambient noise levels during testing. A recent study, where elderly patients were tested in their natural environments using this device and thresholds compared to the same testing done in a sound booth, revealed comparable results within test-retest limits (submitted paper, Mclennan-Smith, Swanepoel, and Hall). At present, the device is employed at a tele-audiology clinic in South Africa as part of a primary ear and hearing telehealth service.
Automated test sequences. To increase test capacity with limited professional resources, the device also incorporates automated test sequences (both conventional pure-tone audiometry and discreet-frequency Bekesy methods). A facilitator can provide a patient with the necessary test instructions or these can be pre-recorded onto the system. Patients use a response button connected to the notebook running the audiometer software that allows automated completion of air and bone conduction audiometry (Figure 3). The results can subsequently be uploaded to a secure server for interpretation by remote hearing care professionals. The automated test sequences utilized by the KUDUwave audiometer have been validated against manual audiometry showing variability within accepted test-retest limits.11 These automated test sequences are used as the primary means for first diagnostic appointments at the pilot tele-audiology clinic in South Africa. For patients who present with unreliable findings, remote synchronous assessments are scheduled.
Remote audiometry. A remote hearing care professional can conduct audiometry on patients who are difficult-to-test or who present with unreliable findings using automated audiometry. As long as the notebook computer controlling the KUDUwave audiometer is connected to the Internet (using a 3G connection at the South African clinic for example), the clinician can take control of the device using desktop-sharing software while reinstructing the patient using videoconferencing software.
The KUDUwave software package also includes novel visual feedback cues to facilitate valid testing, even when limited bandwidth results in time lags between stimulus presentation and remote patient responses. As illustrated in Figure 2, the software provides a green marking on the audiogram at the frequency and intensity where a patient pressed the response button within 1.5 seconds after stimulus presentation. If a patient does not respond within that time, an orange marking appears indicating a no-response. If there was a no-response while the noise was non-compliant, a yellow marking appears to indicate a no-response but in noise levels that may have masked the response.
Using visual feedback cues, the clinician can see the patient’s responses on-screen in making decisions during the test, and this may serve as quality control information for later reviews of test findings. Remote testing with the device has been validated in an intercontinental test setup with subjects in South Africa and a remote audiologist in Dallas, Tex. No significant difference between thresholds in the face-to-face compared to the remote test setup was found.12
Conclusion
The need for diagnostic audiometry is far greater than the current capacity of hearing healthcare professionals to deliver these services. The rapid growth in global connectivity, however, is opening up access possibilities for delivering hearing healthcare using telehealth models.
Capitalizing on these developments requires rethinking current audiological practices and being innovative with technology. The gold standard of hearing diagnosis—pure-tone audiometry—can be improved for tele-audiometry to deliver diagnostic services outside of traditional test situations incorporating automation and remote testing to increase audiological capacity and reach. Ultimately, the goal is to harness the growth in technology and connectivity to ensure more people with hearing loss have access to the benefit of hearing health services globally.
CORRESPONDENCE can be addressed to:
References
- World Health Organization (WHO). Deafness and hearing impairment. Geneva: World Health Organization; 2006: Available at: www.who.int/mediacentre/factsheets/fs300/en/index.html
- Stevens G, Flaxman S, Brunskill E, Mascarenhas M, Mathers CD, Finucane M. Global and regional hearing impairment prevalence: An analysis of 42 studies in 29 countries. Eur J Public Health. 2011; Dec 24. Available at: www.ncbi.nlm.nih.gov/pubmed/22197756
- Fagan JJ, Jacobs M. Survey of ENT services in Africa: Need for a comprehensive intervention. Glob Health Action. 2009;2:1-8. Available at: www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2779942&tool=pmcentrez&rendertype=abstract. Accessed July 31, 2011.
- Goulios H, Patuzzi RB. Audiology education and practice from an international perspective. Int J Audiol. 2008;47:647-664.
- Margolis RH, Morgan DE. Automated pure-tone audiometry: an analysis of capacity, need, and benefit. Am J Audiol. 2008;17:109-113.
- Swanepoel DW, Clark JL, Koekemoer D, et al. Telehealth in audiology: The need and potential to reach underserved communities. Int J Audiol. 2010;49:195-202.
- Swanepoel DW, Olusanya BO, Mars M. Hearing health-care delivery in sub-Saharan Africa—a role for tele-audiology. J Telemed Telecare. 2010;16:53-56.
- Swanepoel DW, Biagio L. Validity of diagnostic computer-based Air and forehead bone conduction audiometry. J Occup Environ Hyg. 2011;8:210-214.
- Franks JR. Hearing measurement. In: Goelzer B, Hansen C, Sehrndt G, eds. Dortmund, Germany: World Health Organization, Federal Institute for Occupational Safety and Health; 2001:183-232.
- Berger EH, Kieper RW, Gauger D. Hearing protection: Surpassing the limits to attenuation imposed by bone-conduction pathways. J Acoust Soc Am. 2003;114:1955-1967.
- Swanepoel DW, Mngemane S, Molemong S, Mkwanazi H, Tutshini S. Hearing assessment—reliability, accuracy, and efficiency of automated audiometry. Telemed J e-Health. 2010;16:557-563.
- Swanepoel DW, Koekemoer D, Clark J. Intercontinental hearing assessment—a study in tele-audiology. J Telemed Telecare. 2010;16(5):248-252.
ALSO IN THIS SPECIAL ISSUE (OCTOBER 2012) ON TELEAUDIOLOGY:
- Extending Hearing Healthcare: Tele-audiology, by Jerry Northern, PhD
- The Need for Tele-audiometry, by De Wet Swanepoel, PhD
- Are You Ready for Remote Hearing Aid Programming? By Jason Galster, PhD, and Harvey Abrams, PhD
- Infant Diagnostic Evaluations Using Tele-audiology, by Deborah Hayes, PhD
- Online Global Education and Training, by Richard E. Gans, PhD
- Therapeutic Patient Education via Tele-audiology: Brazilian Experiences, Deborah Viviane Ferrari, PhD
- Telepractice in the Department of Veterans Affairs, by Kyle C. Dennis, PhD, Chad F. Gladden, AuD, and Colleen M. Noe, PhD
Citation for this article:
Swanepoel DW. The Need for Tele-audiometry Hearing Review. 2012;19(10):18-25.