University Hospitals to Participate in Clinical Trial for Cochlear Implants
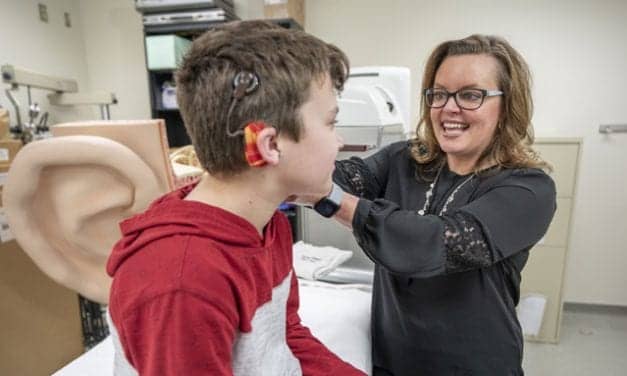
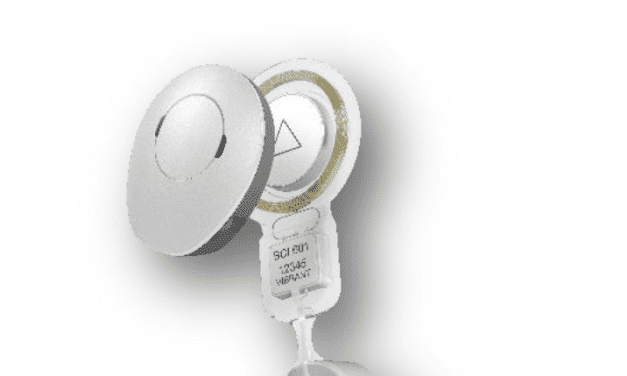
University Hospitals Cleveland Medical Center and UH Rainbow Babies and Children’s Hospital are participating in a Cochlear Americas’-sponsored clinical trial for an implantable hearing device in children 5 to 11 years of age who have been born with hearing loss that may be caused by craniofacial abnormalities.