Some of the latest hearing industry news from HearingReview.com.
- ReSound Survey: iPad App Helps Dispensers Sell More Hearing Aids
- Books: How Hearing Loss Impacts Relationships—Motivating Your Loved One
- Vivosonic Introduces Integrity V500 ABR “Lite” for ENT Clinics
- Mutant Zebra Fish May Help Discover Cause of Fraser Syndrome Hearing Loss
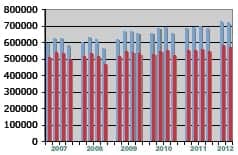
- Report: US Hearing Aid Market Potential Remains High, But Cochlear Implants May Catch Up
- GN Otometrics Announces New Distribution Strategy
- Delaware Governor Markell Signs Two Hearing Loss Related Bills into Law
Six Hearing Aid Manufacturers Form Hearing Research Consortium

The hearing industry’s largest manufacturers have collaborated to create the Hearing Industry Research Consortium (IRC). The IRC’s mission is reportedly to develop and direct a noncompetitive, mutually agreed upon research agenda that is designed to benefit the industry, as well as hearing professionals and end users.
The IRC board is comprised of the heads of research from GN ReSound, Oticon, Phonak, Siemens, Starkey Hearing Technologies, and Widex, including GN ReSound’s Andrew Dittberner; Oticon’s Graham Naylor; Phonak’s Stefan Launer; Siemens’ Joel Beilin; Starkey Hearing Technologies’ Brent Edwards; and Widex’s Lars Sunesen.
As its first act, the IRC is requesting proposals for a research project on the interaction of cognition and hearing aids. The $300,000 grant was announced at the International Hearing Aid Research Conference in Lake Tahoe, Calif, and is open to research labs around the world.
Applications are available on the IRC Web site at www.hearingirc.com/#requests. The deadline for proposals is November 1, 2012, and decisions will be announced by December 31, 2012.
40 Enroll in Phase II Clinical Trial of Auris Medical’s Tinnitus Treatment
Auris Medical has announced that enrollment has been completed for the first of two stages in its TACTT1 phase II clinical trial with AM-101 for the treatment of acute inner ear tinnitus.
A total of 40 patients were enrolled in the study and received either AM-101 at 0.81 mg/mL or placebo in a single dose intratympanic injection.
The purpose of the TACTT1 study is to further evaluate the optimum dosing regimen for AM-101, as well as to generate additional safety and pharmacokinetic data. It is being conducted in the United States, Belgium, Germany, and Poland.
It has been hypothesized that the upregulation of N-methyl-D-aspartate (NMDA) receptors induced by cochlear excitotoxicity is responsible for aberrant excitation of auditory nerve fibers, which is perceived as tinnitus.
According to Auris Medical, AM-101 contains a small molecule designed to selectively block NMDA receptors. Emerging evidence suggests that these receptors in the cochlea play a major role in the occurrence of tinnitus following inner ear excitotoxicity, which is characterized by excessive synaptic release of glutamate, the principal neurotransmitter in the auditory system.
HLAA Announces Annual Awards
Hearing Loss Association of America (HLAA) presented its annual national awards during its 2012 Convention. Among the recipients:
The Walter T. Ridder Award was presented to David G. Myers, PhD, for his “Let’s Loop America Campaign” and for increasing awareness about communication access through his writings (which include HR) and ability to attract national media attention.
The Howard E. “Rocky” Stone Humanitarian Award was given to Bea Lyons of Chattanooga, Tenn. The award honors an outgoing or past trustee for extraordinary contributions toward the furtherance of the objectives and personal exemplification of the philosophy envisioned by Howard E. Stone, founder of the organization.
The National Access Award was presented to Brown University, which was recognized for its disability access services, especially those with hearing loss, to participate fully in the programs that the university offers.
Download a full list of all the HLAA 2012 award recipients at ow.ly/cZszz.