Dispensing professionals are aware of the critical need for comprehensive clinical testing of any new product or algorithm. The leading hearing instrument manufacturing companies routinely perform clinical testing on their products. In this way, there is a commitment to formal clinical procedures/policies to ensure that products and software have been thoroughly tested prior to their introduction. For example, clinical research at GN ReSound is carried out in three stages: 1) research testing; 2) formal alpha-testing in-house (in Chicago, in Eindhoven, Holland, and in Copenhagen, Denmark), and 3) formal beta testing (worldwide). All three of these stages focus on how the hearing-impaired subjects benefit and perform (subjectively and objectively) with the new products or fitting systems.
The following article describes the U.S. and worldwide beta test of the GN ReSound Canta7 Series. This hearing instrument is the first new product line and fitting software for GN ReSound since the consolidation of ReSound and GN Danavox by GN Great Nordic, and it represents the first “3D hearing instrument family” developed by the combined resources of the companies.
Design Rationale
The digital technology of Canta7 combines GN ReSound’s existing compression system with a LASER system of fast noise reduction and spectral enhancement, adaptive directionality, and adaptive digital feedback suppression—all of which are designed to automatically adjust to the changing environment.
Like the company’s previous products, the foundation for the new instrument’s compression system is Digital Cochlea Dynamics™ processing.1 The instrument uses a 64-band Fast Fourier Transform (FFT) system to create a set of 14 overlapping frequency bands corresponding to the function of the cochlea. Like a healthy cochlea, the dynamic range of the compression system has to cover the complete dynamic range of human speech. To achieve this, the compression kneepoints are set below the level of soft speech. Coupled with syllabic compression and fast-acting time constants, the algorithm is designed to adjust quickly to constantly changing input levels, closely matching the compression system of a healthy cochlea.
Feedback problems are managed within the instrument by the Adaptive Digital Feedback Suppression (DFS)2,3 algorithm. The DFS uses a “search and destroy” digital technique for detecting feedback and eliminating it; therefore, rather that reducing gain, it actually can provide up to 10 dB of extra gain in what might otherwise be considered a problem fitting.
To help manage background noise problems, the LASER system1,3,4 was developed. This system has two algorithms to choose from: spectral enhancement or noise reduction. The spectral enhancement algorithm manipulates every point in the speech spectrum so that spectral peaks are emphasized and spectral valleys are reduced, keeping overall loudness constant. A new 64-band frequency spectrum is calculated using FFT analysis every 4 milliseconds. In effect, this is like stretching the frequency spectrum vertically without changing the overall loudness.
The 14-band noise reduction system continually “listens” to sounds in the user’s environment and tries to determine whether individual sounds are speech, background noise or a mix of both by evaluating the modulation of the signal in all the bands. The intended effect is that the loudness of background noise stays reduced before, during and after the speech while speech remains unaffected. The noise reduction is designed to work so fast that it not only reduces background noise when there is no speech but it also reduces background noise in the tiny pauses between syllables or words.
Additionally, the instrument’s Adaptive Directionality3,4 analyzes the environment (receiving input from both microphones) and updates a directional polar pattern as often as 250 times per second. The effect is to create an instant polar pattern—hypercardioid, cardioid, bi-directional or anything in between—for any situation. This results in the reduction of the loudest signals from the side and rear hemispheres. Active microphone matching (AMM) constantly monitors the output from both microphones to ensure that the microphones are digitally matched over the lifetime of the hearing instrument.
Beta Clinical Trial
Beta testing of the Canta Series and Aventa fitting software began in January 2001 and was completed 10 weeks later. Seventeen U.S. sites, six European sites and one Brazilian site participated, and a total of 130 subjects (255 devices) were fit with the instruments. All of the beta sites were required to follow a formal protocol that included subjective and objective testing. Five different devices models were tested: 710 (CIC), 730 (ITC), 740-D (directional half shell), 750-D (directional ITE), 770-D (directional BTE), 780-D (directional power BTE, referred to hereafter as PBTE).
Objectives: The main objectives of this study were: 1) to validate that the fitting algorithms provide user benefit with a limited amount of fine-tuning; 2) to ensure that the devices perform as well as, or better, than the reference devices on a variety of objective and subjective measures; 3) to evaluate custom device fit and cosmetics, and 4) to ensure stable performance of the devices and the new fitting software. In addition, in the PBTE study, the performance of the multi-function push-button and Power-Fit algorithm were evaluated.
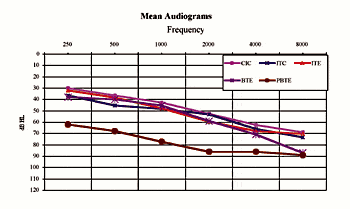
Subjects: Subjects wearing the custom products and BTEs demonstrated mild/moderate-to-severe sensorineural hearing losses (Fig. 1). Subjects participating in the PBTE test demonstrated severe-to-profound hearing losses. The mean age of the subjects (those that reported) was 64.5 years old (range: ages 9-91), and one-third of the subjects were female and two-thirds male. Nearly all (97%) of subjects were prior hearing instrument users.

|
| Fig. 1. Mean hearing loss of subjects grouped by device model. |
Protocol: Subjects were required to attend at least three sessions following the taking of the audiogram and impressions. At the initial fitting session, they were required to answer a questionnaire concerning the functionality of their own devices (i.e., the reference devices in this study) and then they were fit with the new test devices.
The test devices were programmed with the company’s “Audiogram+” fitting algorithm coupled with one of the LASER system choices (spectral enhancement or mild noise reduction). The multi-program devices (ITE, BTE & PBTE) were also programmed with two other presets. Preset 2 contained the new adaptive directionality technology coupled with moderate noise reduction algorithm. Preset 3 was programmed for a telecoil. All subjects utilized the adaptive digital feedback suppression system when needed.
Subjects wore the devices for at least two weeks prior to returning for a fine-tuning session. At this second session, device measurements were completed along with a usage questionnaire. At the final session, approximately two weeks later, subjects completed the initial questionnaire on device functionality with the experimental device, participated in speech in noise objective testing, and made the decision to purchase or return their devices.
Objective Test Results
Speech testing: Speech perception scores were obtained in noise (speech weighted or babble). Recorded monosyllabic word lists were presented at 65 dB SPL with a 0 S/N ratio (+5 S/N ratio for the PBTE). Speech was presented at 0° azimuth and noise at 180° azimuth for four conditions: 1) unaided; 2) reference device; 3) Program 1 consisting of the Audiogram+ with mild noise reduction or spectral enhancement, and 4) Program 2 consisting of adaptive directionality with moderate noise reduction. Mean test results were calculated for each of the device models (Fig. 2).

|
| Fig. 2. Mean scores across devices/subjects for speech perception. |
The subjects performed significantly better in all the aided conditions compared to the unaided conditions. In the CIC model, a significant difference (p<0.05) is noted between the test instrument and the reference device. In the ITE/BTE devices, a significant difference (p<0.05) is noted between Program 2 and Program 1. In the PBTE, scores obtained for Program 1 show a significant improvement over the reference devices, and Program 2 shows a significant improvement over Program 1. With the ITE/BTE and PBTE devices, it was expected that Program 2 (featuring adaptive directionality) would provide significant benefits over Program 1.
Subjective Test Results
Questionnaire: Subjects were asked to rate their own reference devices for various listening situations at the first session and then to rate the Canta7 devices at the final session. The results of the dual microphone ITE and BTE are reported in Fig. 3. Similar results were obtained with the ITC, CIC and PBTE devices. A significant preference (p>0.05) for the test device is noted in all categories except “Telecoil”.

|
| Fig. 3. Test subjects’ ratings for the reference devices and for the ITE and BTE test devices. |
Physical fit/Cosmetics/Reliability/ Stability: On average, clinicians rated the cosmetics and physical fit as good to excellent. In addition, mean ratings of the beta devices were better than comparative devices of the same style. Only four devices (1 ITE, 1 ITC, and 2 CICs) out of 122 custom devices (3%) in the beta study needed to be remade. The reliability and stability of the Canta devices were also good. Overall, only 6 out of 255 devices (2%) were returned for repair. Of these, 4 were BTEs (out of 67 PBTE devices) and 2 were CICs (out of 54 CIC devices).

|
| Fig. 4. Purchase and return rate of each of the test models at the end of the beta trial. |
Purchase rate: Subjects in the U.S. were given the option to purchase their Beta devices at the end of the study. Typically this is a fair indicator to the manufacturer on user acceptance. Dispensers elected to give their subjects between a 0-30% discount (one dispenser gave a 50% discount). As shown in Fig. 4, a majority (71%) of the subjects chose to purchase their test devices. Many of these same subjects were previously wearing high-end digital devices or ReSound analog instruments (Table 1).
|
|||||||||||||||||||||
| Table 1. The percentage of subjects who wore various types of instruments (3% were new users) prior to the beta test and the percentage of these same subjects who elected to purchase the Canta7 test instrument. *Percentages do not add up to 100% due to rounding. |
Conclusion
Clinical testing of any algorithm or product remains an important aspect of research and development for hearing instrument manufacturers. The positive results from this beta test indicate that hearing-impaired subjects perceive and achieve benefit with Canta7 products.
One of the challenges of today’s hearing instrument manufacturer is to get more information on the performance of their new products via laboratory tests, clinical trials and, importantly, in daily use by hearing care professionals. Clinical experiences and case studies involving new products are extremely valuable, and we invite dispensing professionals to share case studies that you think we would find interesting.
This article was submitted to HR by Laurel Olson, MA, research audiologist at GN ReSound, Bloomington, MN. Correspondence can be addressed to HR or Laurel Olson, GN ReSound, 8001 Bloomington Freeway, Bloomington, MN 55420-1036; email: [email protected].
References
1. Edwards B, Struck C, Dharan P & Hou Z. New digital processor for hearing loss compensation based on the auditory system. Hear Jour. 1998;51(8):38-49.
2. Edwards B. Beyond Amplification: Signal processing techniques for improving speech intelligibility in noise with hearing aids. Seminars in Hearing. 2000; 21(2):137-156.
3. Olson L, Musch H & Struck C. Digital Solutions for feedback control. Hearing Review. 2001; 8 (5):44-49.
4. Matsui G & Lemons T. A special report on new digital hearing instrument technology. Hearing Review. 2001;(Suppl); 8 (4):7-31.