Inside Clinical Research | February 2016 Hearing Review
The discovery and management of cognitive issues, which may masquerade as or occur in tandem with hearing problems, allows the professional to better address the global needs of the patient in a timely manner.
It almost goes without saying that one’s cognitive or emotional status, neurologic status, and state of mental health impacts sensory perceptions. The opposite is also true. Specifically, sensory changes can (and do) impact cognitive, emotional, and/or psychological status. Indeed, for the patient with a significant sensory deficit, it’s difficult to imagine their sensory deficit not impacting their cognitive status!
In this paper we advocate for universal cognitive screening of patients 70 years of age and older with hearing loss and/or listening difficulties—even in the absence of obvious signs or symptoms of cognitive impairment.
It is our conviction that improved audiologic outcomes, adherence to aural rehabilitative strategies (including listening strategies and use of assistive listening devices and hearing aids), and improvement in quality of life are the likely outcomes resulting from the incorporation of universal cognitive screenings. That is, the inclusion of cognitive screening tests would improve the ability of the audiologist to better understand and address the very common complaint of not being able to understand speech in noise.
The Interaction of Cognition and Audition
The idea that audiology, cognition, and psychology overlap is not new. Perhaps the earliest exploration and recognition of the overlapping concerns of audiology and psychology was Myklebust1 in 1949 who reported “…for audiology to mature and to become in actuality a science of hearing, it seems necessary that it be influenced more by the fields of special education, psychiatry and clinical psychology…” He continued “…clinical psychology has an important contribution to make in audiology…”
In reality, one cannot readily separate cognition, language, and audition. These processes are intimately interwoven and interdependent. Myklebust stated the most difficult and challenging dilemma is to determine how much of a communicative disorder originates with hearing loss, versus how much is based in other causes. He noted the psychologist is generally concerned with human beings holistically, not hearing loss in particular, while the audiologist is primarily concerned with hearing loss. However, he also noted “that all relative disciplines be applied…as an integrated working whole…” and “The emphasis of clinical psychology would add generally to the field of audiology and to the effectiveness of the audiologist.”
Since Myklebust’s exploration of the overlapping disciplines of audiology and psychology, many others have addressed the interactions of these two disciplines. The National Council on the Aging report titled “The Consequences of Untreated Hearing Loss in Older Persons” published their landmark study.2,3 The authors surveyed some 2,300 adults aged 50 and older with hearing loss in which people with untreated hearing loss were more likely to report anxiety, depression, and paranoia, and participated less in social activities as compared to those with hearing aid amplification. The report again underscored that hearing loss does not occur in a vacuum, and hearing loss may have psychological consequences.
In 2009, Kricos4 reported “One critical area of concern is the need for audiologists to be knowledgeable about age-related cognitive changes that may affect hearing assessment and rehabilitative approaches…” Generally, cognitive changes include executive functions, short-term, long-term, and working memory, the ability to pay attention, as well as the quantity and quality of neural processing. Kricos cautioned that many symptoms of hearing impairment “are identical or similar to some symptoms of cognitive disorders” and these overlapping symptoms “may lead providers to erroneously identify cognitive issues in older patients whose actual problem is hearing impairment…” The opposite problem may occur as well; hearing loss may be suspected when the actual differential diagnosis is founded more in cognitive issues.
A paper by Beck and Clark5 was titled “Audition Matters More as Cognition Declines and Cognition Matters More as Audition Declines” to underscore that interaction and co-dependence of cognition and audition is a paramount concern for audiologists. Indeed, they report when hearing loss is present—that is, when one has a “bottom-up” (sensory) processing impediment (ie, hearing loss)—the “top-down” (cognitive) system has to work harder to fill in (or correct) the missing (or distorted) sounds. They stated “people with hearing loss must dig deeper into their cognitive reserve and abilities to make sense of a world delivered to them via compromised auditory input…”
Beck and Flexer6 reported hearing is the ability to perceive sound, and listening is the ability to make sense of sound (ie, to attribute meaning to sound). Thus “listening is where hearing meets brain.” They note the primary deficit experienced by people with hearing loss is not the inability to hear (a sensory failure) but the inability to assign accurate and correct meaning to sound (a cognitive process).
Lin et al7 concluded “Hearing loss is independently associated with accelerated cognitive decline and incident cognitive impairment in community-dwelling older adults…” Chuan-Ming Li at the National Institute on Deafness and Other Communication Disorders (NIDCD) reported “a strong association between hearing impairment and depression among US adults of all ages, particularly in women…”8
In 2015, Dawes and colleagues9 reported: “Hearing aids may promote better general health, perhaps by reducing hearing handicap and promoting a more active, engaged lifestyle.” Amieva and colleagues (2015) reasoned that by facilitating improved communication, hearing aids may improve mood and increase social interactions, thereby perhaps impacting scores on cognitive tests.
Sensory perception (including hearing, vision, taste, smell and touch) doesn’t occur in isolation. Sensory perceptions occur within the brain, in tandem with internal (within the body) and external (environmental) context. Specifically, top-down (cognitive) and bottom-up (sensory) processes occur simultaneously as they interact with, and impact each other. As such, the inclusion of cognitive screenings (top-down) as an adjunct to audiologic diagnostic testing (bottom-up) for certain populations should be considered.
Universal Cognitive Screenings
The goal of screening for cognitive decline is to uncover the possibility that a patient is at risk for dementia, which in turn, may impact speech understanding and listening ability. Dementia currently affects approximately 4 million Americans with prevalence increasing with age, rising from 5% among persons aged 71 to 79 years, to 37% among those 90 years and over. Another goal of screening for cognitive decline is to identify those with mild cognitive impairment (MCI). MCI differs from dementia in that the cognitive decline associated with MCI is not severe enough to interfere with instrumental activities of daily living.
Of note, universal screening of adults 65+ who do not exhibit signs or symptoms of cognitive impairment is not endorsed in a general primary care setting by the US Preventive Services Task Force.10 In fact, the USPSTF recommended a grade of “I” (insufficient evidence) to determine the balance of benefits and harms of screening activities. The task force indicated it may be important to identify early cognitive impairment, since information regarding cognitive status may help patients make treatment and management decisions, especially given co-morbidities and/or potentially reversible causes of dementia. While not advocating universal screening, the task force stated clinicians should remain alert to early signs of cognitive impairment and evaluate accordingly.
Importantly, symptoms of hearing loss often overlap with those of dementia. Further, as hearing loss has been shown to increase the risk of incident dementia, we advocate for routine cognitive screening of adults 70 years of age and older in light of demonstrable hearing loss or difficulty listening in challenging situations. Fortunately, screening for cognitive status is a required element of the initial Medicare Annual Wellness visit for apparently many of these same reasons.
A number of inexpensive and easy-to-administer cognitive screening tests are available to clinicians interested in screening for in one or more of the following cognitive domains: memory, attention, language, and visuospatial or executive functioning. According to Shulman,11 an ideal cognitive screening test should have the following characteristics:
1) Quick, brief, and easy to administer with minimal training;
2) Well tolerated and acceptable to patients, and easy to score;
3) Relatively independent of culture, language, and education;
4) Have good inter-rater and test-retest reliability;
5) Have high levels of sensitivity and specificity;
6) Have concurrent validity such that scores correlate with measures of severity and other dementia rating scores, and
7) Have positive predictive value (percentage of patients who have a positive score on the screening test and who really have the condition).
Additionally, the clinician must feel comfortable asking the questions included in the scale, and must consider the time available for the cognitive screen. Note that for many patients, a cognitive screen will be experienced as an emotional threat or assault on their self-esteem. Accordingly, it is important to consider the frustration, anxiety, and defensiveness tolerances of the person to whom you are administering the scale. Finally, the scale chosen should be applicable throughout the entire dementia disorder spectrum, reliably discriminating between those with and without dementia.
Cognitive Screening Tests
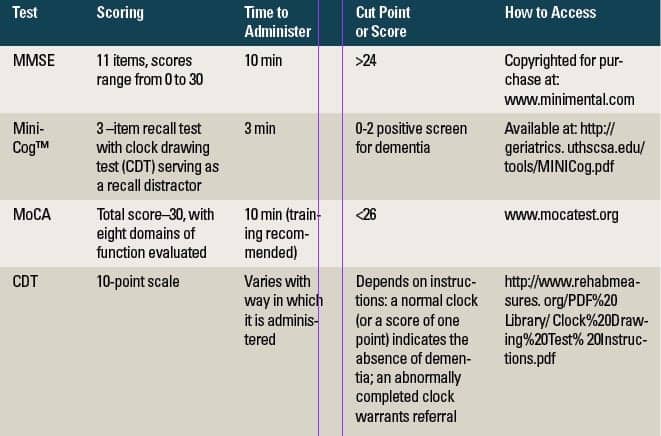
Table 1 outlines and reviews the most widely used cognitive screening tests: the Mini Mental Status Evaluation (MMSE), the Clock Drawing Test, the Mini-Cog Test and the Montreal Cognitive Screening Assessment (MoCA).10
The simplicity of the MMSE may be an advantage for use by audiologists. The MMSE may be the ideal screening test of general cognitive ability; however, it is not sensitive to mild cases of dementia. Scores on the MMSE correlate well with functional capacity, especially the ability to carry out Instrumental Activities of Daily Living (IADL). Note, the MMSE is designed for people who are fluent in English and have at least a grade 8 education.With a sensitivity of 88.3% and specificity of 86.2%, the MMSE is the most widely used screening test with commonly reported “cut points” indicative of risk for dementia being either 23/24 or 24/25. The MMSE is mainly a verbal test used to screen for orientation to place, registration, recall, calculation and attention, naming, repetition, comprehension, reading, writing, language and drawing).12 The maximum total score on the MMSE is 30 with severity of dementia being classified into three categories: 1) a score of 24-30 indicative of no cognitive impairment; 2) 18-23 indicative of mild cognitive impairment, and 3) 0-17 severe cognitive impairment.13 Test/retest reliability coefficients are high and internal consistency (while acceptable) is not as high as the former coefficient.
The Mini-Cog™ combines a test of recall with a clock drawing test.14,15 These include language comprehension, memory, visual-motor skills, and executive function. The Mini-Cog is brief, sensitive, easy to administer, and scores are not strongly influenced by education or language barriers. It has high sensitivity and specificity, and accurately differentiates persons with and without dementia. Scores correlate well with performance on the MMSE.
The Montreal Cognitive Assessment (MoCA) has been designed as a tool to screen patients who present with mild cognitive complaints and (most often) perform in the normal range on the MMSE. The domains of function evaluated include a short-term memory recall test, a clock drawing test, and a three dimensional cube copy to assess visuospatial abilities, a test of executive function, orientation to time and place, language, abstraction, memory, and delayed recall.16 The MoCA performs well with respect to test/retest reliability and internal consistency and scores correlate well with the MMSE. In contrast to the MMSE, the developers recommend administering the MoCA to persons who present with cognitive complaints and functional impairment—especially if they fail the MMSE. According to Nasreddine et al,16 the MCI is sensitive to mild cognitive impairment, whereas the MMSE is very simple for those with normal cognitive function, but presents a challenge for those with functional impairment and advanced stages of dementia. There are many versions of the MoCA online, including a tablet and a shortened version.
The Clock Drawing Test (CDT) provides a simple and reliable measure of a comprehensive range of cognitive functions, including visuospatial construction ability (a skill known to be impaired in dementia even in early stages), auditory comprehension, planning, executive function, abstract thinking, visual memory, motor programming and execution, numerical knowledge, and abstract thinking.11 It is important to note that interpretation of scores on the CDT also assess clock setting and clock reading. Patients are asked to fill in the numbers and set the time to designated times. The CDT can be administered within 3 minutes, and scoring is quite easy, with points awarded according to components of the clock included. There are a variety of scoring systems and the extent of the correlation with the MMSE varies with the system adopted. The CDT has high reliability, yet psychometric properties vary with the scoring method utilized. The CDT is best used as an adjunct to other cognitive screening tests rather than as a standalone.17 The CDT test appears to be well tolerated, especially for persons with short attention spans.
In summary, numerous reliable and valid tests are available to screen cognitive ability. Nonetheless, prior to including cognitive screenings as part of a global auditory evaluation, it is highly recommended that the audiologist locate, identify, and consult a local psychologist (or other professional counselor) who will be available to work with patients who fail cognitive screenings and are referred for professional evaluation and management. That is, one must prepare in advance, a network of professionals and agencies to maximally address the needs and concerns of patients who fail cognitive screenings, much as we would refer the patient who has medical or surgical indications to a physician.
Explaining Cognitive Screening to a Patient
Many patients and hearing healthcare professional (HHP) will have difficulty introducing cognitive screenings into an audiology practice. One patient recently asked, “Shouldn’t you be evaluating my ears, not my head?”
The HHP should address and validate the patient’s befuddlement, assuage any anxiety, and explain in a user-friendly manner that cognitive and sensory processes are inextricable. With a compassionate smile, the HHP could answer the patient’s question (in the above query) with “I understand your confusion, but don’t worry. You know, the brain not only hears sounds, but it also makes sense out of sound. That is, the brain attributes meaning to sound. All these systems are connected. Of course, you’re right! My primary role is to evaluate, measure, and address the hearing part. Fortunately, I work with other professionals who can help teach your brain to make sense out of sound and to listen better. The cognitive screening helps me better understand how your brain works with sound, and helps me make better recommendations for you concerning your listening difficulty.”
How to Handle Patients Who Fail Cognitive Screenings
Patients who fail cognitive screenings often experience an emotional crisis. Of note, when the word “crisis” is written in Chinese, two characters are used. The first represents danger, and the other represents opportunity. The HHP can facilitate the opportunity aspect in that the HHP is in a pivotal position to reduce patients’ anxiety and shame, and facilitate the expansion of a supportive network of other helping professionals.
When patients fail cognitive screenings, it is important to minimize their anxiety and shame. There are better and worse ways for the HHP to inform the patient of their screening results. For example, recommending further evaluation of cognitive factors generally triggers significantly less anxiety than openly speculating about a cognitive impairment or even dementia.
Additionally, shame occurs when one cannot psychologically differentiate oneself from the impairment. For example, one patient might say “I am affected by dementia” as compared to one who cannot differentiate and might say “I am demented.” Accordingly, when recommending further evaluation, it is helpful to assure patients that their identity and competence are not defined by the results of the screening. If and when a patient fails a cognitive screening, the HHP should reinforce that the patient has many assets which are not affected by their disability and that other professionals (non-HHPs) can teach management and compensatory strategies to help maximize listening skills. Thus, the HHP sets the stage for differentiating the patient’s self-identity from their impairment, thereby reducing shame while ushering in other collaborative professionals.
As previously stated, if the audiologist chooses to pursue cognitive screenings, professional counselors must be available to further evaluate and manage identified patients. Ideally, the mental health professionals will have more than familiarity, and will have dual competencies in treating/managing people with hearing loss and treating/managing people with cognitive impairment. Professionals without such expertise may do significant psychological harm by falsely minimizing and/or maximizing the effects of these disabilities. Unless a referral is made in a careful and compassionate manner, to a knowledgeable professional, it will likely result in non-adherence and may damage your relationship.
How to Refer an Audiology Patient for Mental Health Services
The following are guidelines for the HHP to make a successful mental health referral (for more details, see Harvey18):
Elicit, contain, and validate the patient’s feelings. Open-ended questioning is an effective way of conveying to patients your appreciation of their emotional reactions while gently setting limits. Of note, without limits, an audiologic visit may spin out of control and may open a can of “emotional worms.”
Normalize (de-stigmatize) the referral. When first discussing a referral, do not use loaded words such as “psychotherapist” or “psychologist.” Although well intentioned, these words may be experienced by patients as assaults to their self-esteem. Instead, it is important for the HHP to support a patient’s self-competence while initiating a referral. Empowerment is the antithesis of anxiety and shame. For example:
“There are many communication skills people with hearing, listening and memory problems learn to use. I believe you can learn them quite readily. Can I refer you to Dr Smith, who can spend some time with you and teach you those tools?” And you might add, “I’ve found it more successful to use a holistic, team approach to help people manage this.”
Humanize the mental health professional. This is an effective method of reducing anticipatory anxiety. For example:
“I’ve known Dr Smith for over 20 years. She’s nice, excellent at what she does, and has been practicing psychology for over 30 years. She has a dry sense of humor and wonderful manner, according to my patients whom I refer to her . I think you’ll like her.”
How the psychologist will approach the patient, and exactly what the psychologist will do will vary with the specific patient, the psychologist, and the primary issues to be addressed. However, the psychologist will very likely conduct a thorough psychodiagnostic assessment of the patient’s cognitive and emotional functioning.
Conclusion
Cognition, language, psychology, audition (and more) are integrated and intertwined. As we move deeper into the 21st century, the time has come to consider the impact of hearing loss and listening problems globally. That is, we certainly know that the impact of hearing loss extends far beyond elevated pure-tone thresholds, and hearing loss clearly impacts emotions, moods, psychological status, quality of life, daily function, and so much more.
Therefore, we advocate universal cognitive screening of patients 70 years of age and older with hearing loss and/or listening difficulties—even in the absence of obvious signs or symptoms of cognitive impairment. The discovery and management of cognitive issues, which may masquerade as or occur in tandem with hearing problems, allows the professional to better address the global needs of the patient in a timely manner.
References
-
Myklebust HR. The relationship between clinical psychology and audiology. J Sp Hear Disord. 1949; 14(6):98-103. doi:10.1044/jshd.1402.98
-
National Council on the Aging (NCOA). The Consequences of Untreated Hearing Loss in Older Persons. Available at: https://www.ncoa.org/wp-content/uploads/NCOA-Study-1999.pdf
-
Kochkin S, Rogin C. Quantifying the obvious: the impact of hearing aids on quality of life. Hearing Review. 2000;7(1):8-34. Available at: http://www.betterhearing.org/pdfs/Hearing_aids_and_quality_of_life_NCOA.pdf
-
Kricos P. Providing hearing rehabilitation to people with dementia presents unique challenges. Hear Jour. 2009; 62(11):39-43. Available at: http://journals.lww.com/thehearingjournal/Fulltext/2009/11000/Providing_hearing_rehabilitation_to_people_with.9.aspx
-
Beck DL, Clark, JL. Audition matters more as cognition declines and cognition matters more as audition declines. Audiology Today. 2009;(3):48-59.
-
Beck DL, Flexer C. Listening is where hearing meets brain in children and adults. Hearing Review. 2011;18(2):30-35. Available at: https://hearingreview.com/2011/10/listening-is-where-hearing-meets-brain-in-children-and-adults-2/
Lin FR, Yaffe K, Xia J, et al. Hearing loss and cognitive decline in older adults. JAMA Intern Med.2013;173(4):293-299.doi:10.1001/jamainternmed. 2013.1868. http://archinte.jamanetwork.com/article.aspx?articleid=1558452
-
Li CM. NIDCD Researchers find strong link between hearing loss and depression in adults. March 7, 2014. http://www.nidcd.nih.gov/news/releases/14/Pages/030714.aspx
-
Dawes P, Cruickshanks K, Fischer M, et al. Hearing aid use and long-term health outcomes—Hearing handicap, mental health, social engagement, cognitive function, physical health and mortality. Int J Audiol. 2015;54:838-844.
-
Lin J, O’Connor E, Rossom R, et al. Screening for cognitive impairment in older adults: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2013;159:601-12.
-
Shulman K. Clock-drawing: Is it the ideal cognitive screening test? Int J Geriatric Psychiatry. 2000;15: 548-561.
-
Folstein M, Folstein S, McHugh P. Mini-mental state: a practical method for grading the cognitive state of patients for the clinician. J Psychiatric Res. 1975;12:189-198.
-
Tombaugh T, McIntyre N. The Mini-Mental State Examination: A comprehensive review. J Am Geriatr Soc. 1992;40:922–935.
-
Borson S, Scanlan JM, Watanabe J, Tu SP, Lessig M. Improving identification of cognitive impairment in primary care. International J Geriatr Psychiatry. 2006;21:349-355.
-
Borson S, Scanlan M, Chen P, et al. The Mini-Cog as a screen for dementia: validation in a population-based sample. J Am Geriatr Soc. 2003;51:1451–4
-
Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S., Whitehead V, Collin I, Cummings JL, Chertkow H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699.
-
Kørner E, Lauritzen L, Nilsson F, Lolk A, Christensen P. Danish Medical Journal. Simple scoring of the Clock-Drawing Test for dementia screening. Accessed December 24, 2015. http://www.danmedbul.dk/portal/page/portal/danmedbul.dk/dmb_forside/PAST_ISSUE/2012/DMJ_2012_01/A4365/A4365.pdf
-
Harvey MA. How to refer patients successfully to mental health professionals. Hearing Review. 2008;15(7):20-24. Available at: https://hearingreview.com/2008/07/how-to-refer-patients-successfully-to-mental-health-professionals
Douglas L. Beck, AuD, is Senior Editor of Inside Clinical Research for The Hearing Review and is among the most prolific authors in audiology. In addition to his new duties at HR, Dr Beck is an Associate Professor of Audiology at the University of Hawaii and Director of Professional Relations at Oticon Inc, Somerset, NJ.
Barbara E. Weinstein, PhD, is Professor & Founding Executive Officer of the Doctor of Audiology Program at City University of New York.
Michael Harvey, PhD, ABPP, is a clinical psychologist who specializes in issues related to hearing healthcare and also has a psychology practice in Framingham, Mass.
Correspondence can be addressed to HR or Dr Beck at: [email protected]
Original citation for this article: Beck DL, Weinstein BE, Harvey M. Issues in Cognitive Screenings by Audiologists. Hearing Review. 2016;23(2):36.?