Research | September 2020 Hearing Review
Analysis and observations relating to cognitive resource reallocation in hearing loss
In previous research using neural imaging, Hannah Glick and Anu Sharma1,2 showed that subjects who attained normal bilaterally aided scores on the Quick Speech in Noise (QuickSIN) test3 appeared to have been spared the cognitive resource reallocation problem observed in patients with lower aided QuickSIN scores. The question is “Why?” What are some of the characteristics of people who obtain normal scores on the QuickSIN? We looked at this question by analyzing a single clinic’s population, where only 63 patients out of a total of 831 persons (7.6%) with similar hearing losses and binaural hearing aid fittings achieved normal scores on the bilaterally-aided QuickSIN in a 2-year period between 2018 and 2020. The purpose of this study, therefore, is to discuss findings of objective/subjective variables associated with these 63 subjects.
By Ron J. Leavitt, AuD, Carol Flexer, PhD, and A. Nikki Clark
Much attention has been given to health conditions that coexist with hearing loss. Specifically, Lin and colleagues4 identified an increased likelihood of falls (see Jiam et al5 for meta-analysis of studies on this topic), increased likelihood of all incident dementia6 (see Nixon et al7 for a literature review), more frequent hospitalizations,8 accelerated shrinkage of the cortex,9,10 and depression.11,12
Glick and Sharma1,2 found that subjects who score normally under bilaterally aided conditions on QuickSIN test show brain resource allocation typical of subjects with normal hearing who perform the same task.By contrast, those with untreated hearing loss or poorer scores on the bilaterally aided QuickSIN show atypical physiologic responses in the temporal, frontal, and prefrontal lobes.
Glick and Sharma2 also noted that more extensive cross-modal recruitment of the right auditory cortex was associated with greater degrees of hearing loss, poorer speech perception in noise, and worse cognitive function. Such cross-modal recruitment was also seen in subjects with mild untreated hearing loss, but to a lesser degree. They hypothesized that recovery of a person’s auditory speech perception abilities after clinical intervention (eg, hearing aid use) may be limited by the extent to which the auditory cortex of someone who has age-related hearing loss (ARHL) has become “re-purposed” by vision. This might serve as a caution that adults with ARHL who wait the average of 7-10 years after hearing loss may be at a disadvantage; for such patients, introducing new auditory stimuli to a central auditory system that has extensively re-organized could potentially limit positive treatment effects and/or make hearing rehabilitation more difficult.2
In addition to the cognitive resource reallocation and cognitive deficits noted above, several other pathophysiological conditions have been associated with hearing loss in multiple central auditory regions, such as the cochlear nuclei, inferior colliculi, thalamus, and primary auditory cortex and portions of the cerebellum that communicate with other cortical networks.13-15
Speck et al15 showed significant regional glucose metabolism of both the inferior colliculus (IC) and primary auditory cortex (PAC) on the contralateral side of the poorer-hearing ear supporting the hypothesis that longer duration of hearing impairment is associated with a higher glucose metabolism, which indicates greater cognitive resource reallocation (see March 2020 news/video16).
Serving as the impetus for the present study, Glick and Sharma suggested, “Future studies should seek to understand differences in individual characteristics and demographic variables that may affect cortical and cognitive outcomes following intervention for adults with ARHL. Such information may help inform best-practice guidelines and help guide clinical recommendations.”2
With the foregoing context in mind, this paper reports findings on objective/subjective variables associated with subjects who scored normally on the bilaterally aided QuickSIN.
Methods
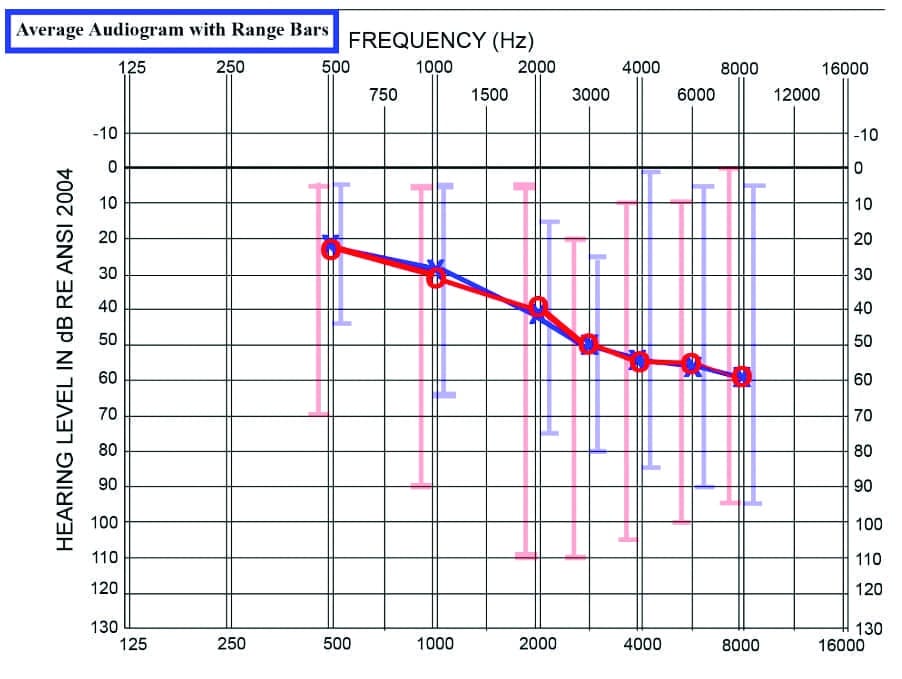
This is a single-site, single-blinded, prospective study. From a single clinic’s population, 63 patients with binaural hearing aid fittings achieved normal scores on the bilaterally-aided QuickSIN in a 2-year period, between 2018 and 2020. There were 26 females and 37 males in this population, ranging in age from 8-82 years, with an average age of 59.7 years, and all wore slim-tube or receiver-in-the-canal (RIC) hearing aids from five different manufacturers with hearing aids ranging in age from 1 day to 15 years. Subjects received pure-tone air/bone conduction threshold testing from 250 to 8000/4000 Hz using the Hughson-Westlake adaptation of the Carhart-Jerger threshold procedure.17 The average audiogram with range bars is shown in Figure 1.
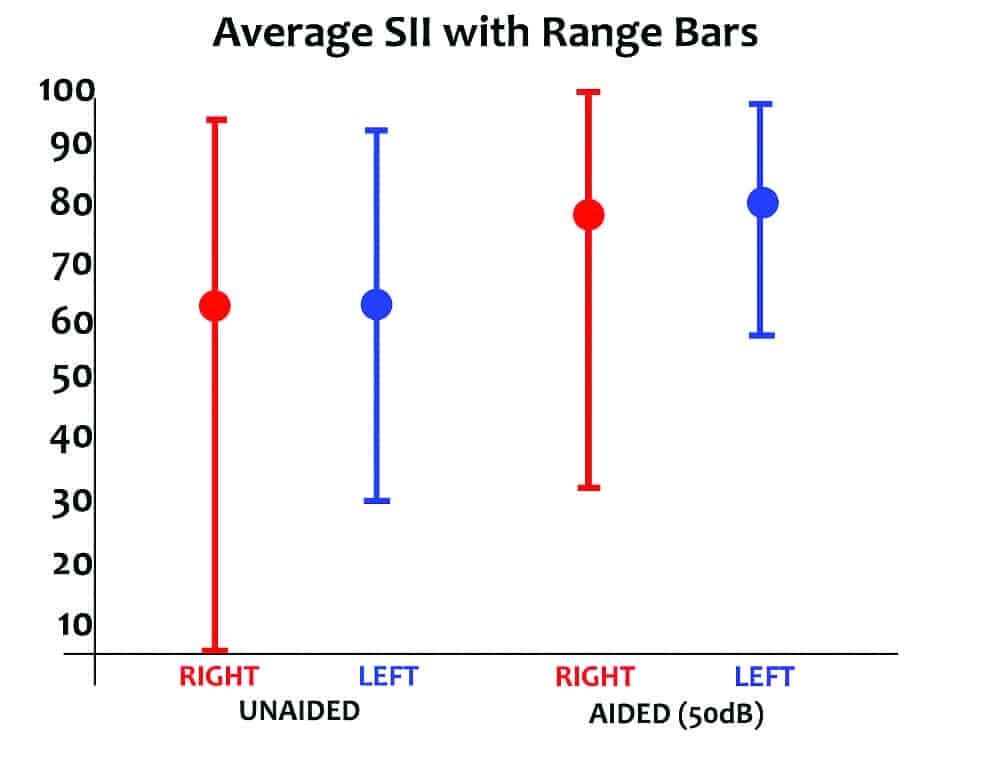
The average unaided and aided Speech Intelligibility Index (SII) of these subjects is shown in Figure 2 per ANSI/ASA S3.5-1997.18
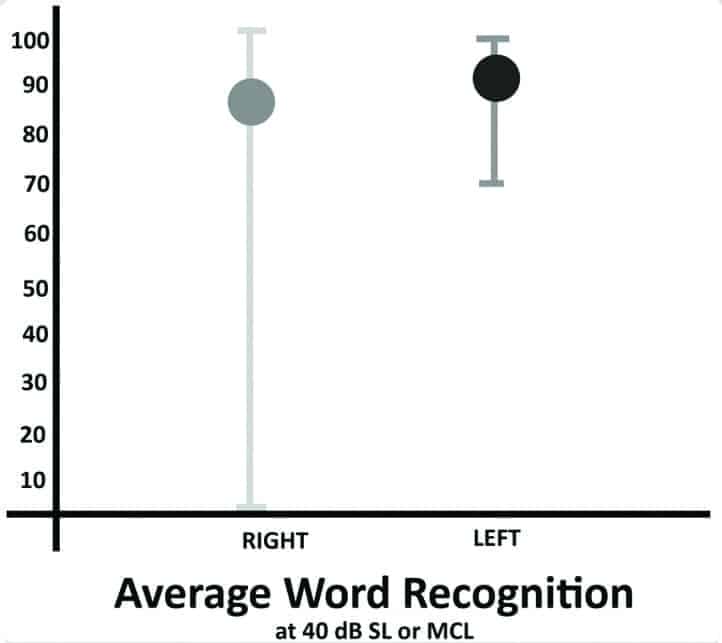
Unaided speech thresholds were obtained bilaterally using the same threshold searching procedure for pure-tones via a recorded version of the W2-word list.19 All subjects received the following tests: immittance with ipsilateral/contralateral reflexes using a GSI 38 Auto Tymp; Olsen Noffsinger tone decay tests at 2000 Hz and 500/1000 Hz as indicated by 2000 Hz results; word recognition testing at 40 dB SL or MCL using a 50-word recorded list of the Maryland CNC, and pure-tone measurements of uncomfortable listening levels between 250 and 4000 Hz using instructions recommended by Cox and colleagues.20-22 The average unaided word recognition scores obtained under earphones are shown in Figure 3.
A 12-frequency distortion-product otoacoustic emission (DPOAE’s) test was performed for both ears using an Interacoustics Otoread device. In all cases, DPOAE results were consistent with pure-tone findings.
No subject had a medically treatable ear condition, flat tympanogram, known fluctuating or rapidly progressing hearing loss, cognitive, medical, or language-based conditions that limited ability to complete all test procedures. No subject had current/recent use of any platinum-based cancer drugs or mycin-family antibiotics, previous diagnosis of multiple sclerosis or Meniere’s disease, or poor speech threshold/PTA/DPOAE agreement.
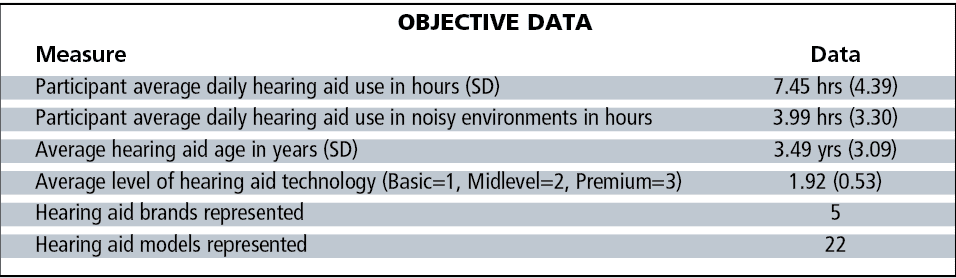
Subject demographics are shown in Table 1. All hearing hearing aids incorporated two directional microphones and a variety of sound processing strategies, ranging from basic to premium levels of technology as described by each manufacturer. A total of 36 of 63 pairs of hearing aids (57%) incorporated wireless connectivity. Tables 2-3 show the objective data on these 63 subjects, as well as their group audiological and hearing aid data.
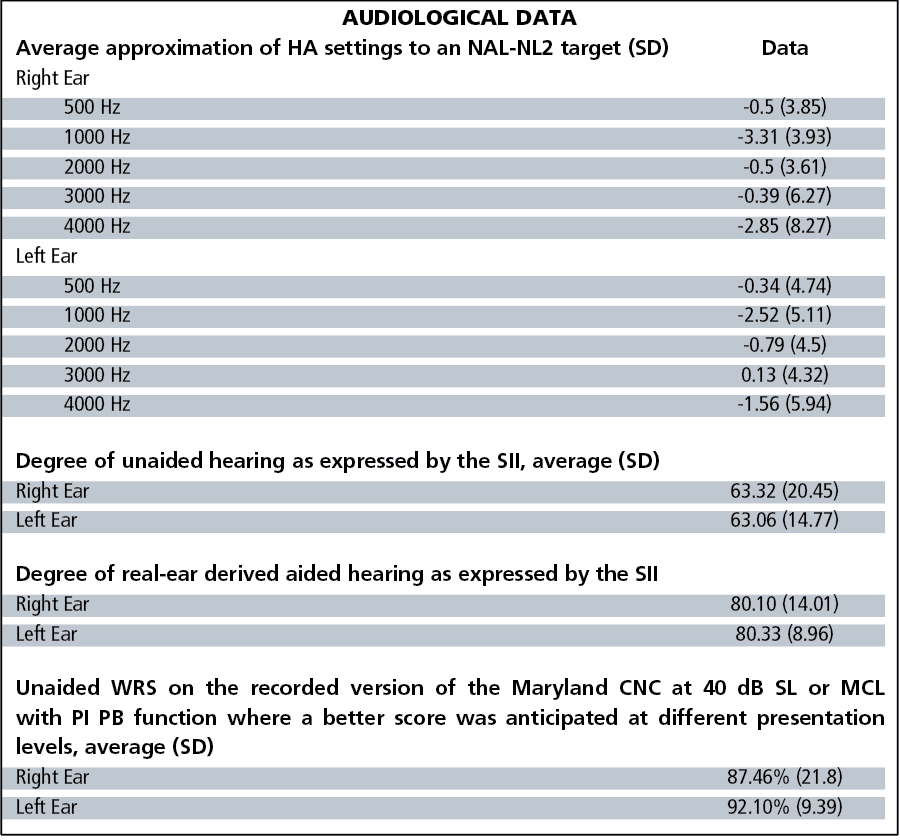
In the bilaterally aided condition, each subject wore their own hearing aids and listened to one practice list and two equivalent lists of the QuickSIN, using equivalent lists as described by McArdle and Wilson.23 All subjects wore their hearing aids set to approximate an average NAL NL-2 target immediately before aided QuickSIN testing.24 The average approximation to an NAL NL-2 target is shown in Figure 4.
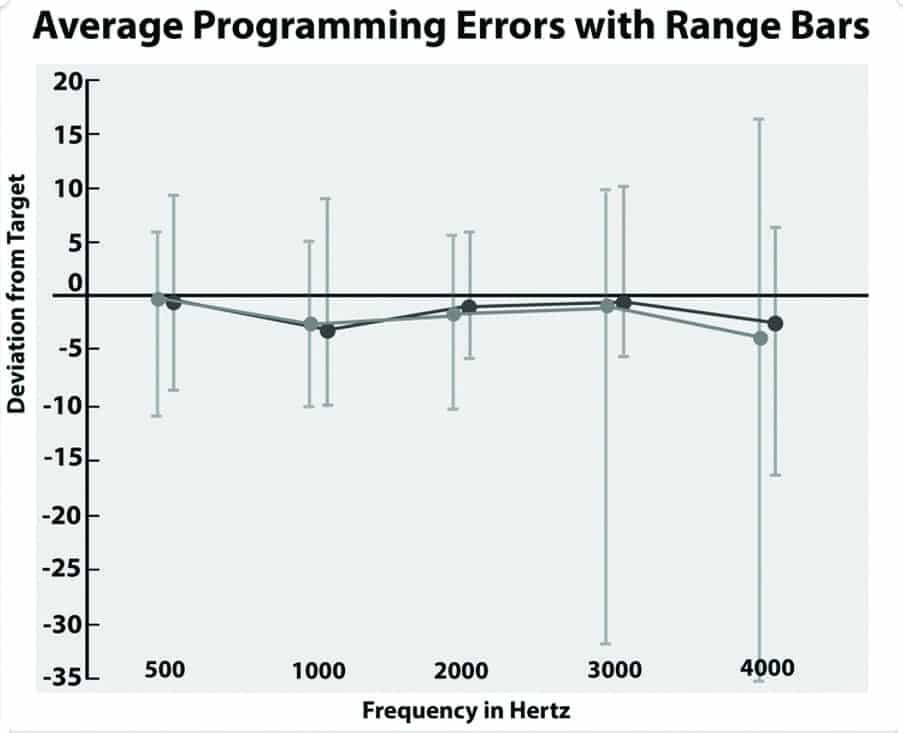
The NAL target was confirmed with an Audioscan Verifit real-ear system, running software version c2.12.1 utilizing the “Carrot Passage” presented at 50-, 60-, and 75-dB SPL. As suggested by the range bars in Figure 4, not all users achieved an NAL NL-2 target due to one large asymmetric hearing loss or insufficient aided gain, feedback, or intolerance of the aided audibility provided by an NAL NL-2 target. Four subjects preferred more gain than suggested by the NAL target, with average deviations between +1 and +3 dB.
To assume identical QuickSIN list equivalency, the same practice list and the same two test lists were used on every subject. In addition, electroacoustic measurements were performed on each hearing aid with an HA-1 2cc coupler in the Verifit test box to assure proper hearing aid function prior to aided testing in noise.25
QuickSIN sentences were presented in the bilaterally aided condition at 50 dB HL in calibrated sound field from a GSI 61 2-channel diagnostic audiometer, with QuickSIN lists presented through a JBL Control I Pro loudspeaker located 1 meter in front (0° azimuth) of the test subjects’ head. The subjects were seated in a Tracoustics sound-insolation booth. All hearing aid batteries, microphone inlets, and receivers were cleaned and/or replaced as necessary immediately prior to aided testing. A normal score on the bilaterally aided QuickSIN was defined as a 2-list averaged score of +1.5 to -3.5 dB SNR loss at the 50 dB HL presentation level in calibrated sound field.
Information about the following 12 subject variables was obtained in a face-to-face interview/evaluation with the lead author:
- Subject age;
- Subject income level;
- Subject educational level;
- Duration of hearing loss;
- Unaided word recognition scores on the recorded version of the Maryland CNC at 40 dB Sensation Level (SL) or Most Comfortable Listening Level (MCL) with PI PB function;
- Mini Mental State Exam Score (except for 8-year-old);
- Degree of unaided and real-ear derived aided hearing as expressed by the Speech Intelligibility Index (SII);18
- Number of hours of daily hearing aid use in noise;
- Type, brand, age, and model of hearing aid;
- Approximation of hearing aid to NAL-NL2 target;
- Level of hearing aid technology (basic to premium inclusive), and
- Hours of daily hearing aid use (obtained on 60/63 pairs of hearing aids incorporating datalogging).
Results
Review of the data revealed that 3 of the 12 variables were consistently associated with normal scores on the bilaterally aided QuickSIN test. Specifically, all 63 subjects had:
- Intact cognitive abilities as measured by the MMSE;
- Good-to-excellent unaided word recognition scores in quiet on the recorded version of the Maryland CNC 50-word list presented at 40 dB SL or MCL;
- Hearing aid fitting proximity to an NAL NL-2 target.
Also noteworthy are the variables not associated with these normal scores. Specifically, neither age of the hearing aid, brand/model, or features of the hearing aid appeared to be significant contributors to these normal scores. There were 9 pairs of basic hearing aids, 46 pairs of mid-level hearing aids, and 8 pairs premium-level hearing aids worn by these 63 subjects, with hearing aid age ranging from 1 day to 15 years, with mean hearing aid age of 3.49 years.
Discussion
These findings are consistent with previous reports showing that neither clinical findings nor hearing aid user self-report differentiates basic-level from premium-level hearing aids in a variety of listening situations when all are programmed to an NAL NL-1/2 real-ear aided response.26,27 Similarly, there was no consistent relationship among level of technology or age/brand/model of hearing aid and normal bilaterally aided scores on the QuickSIN, provided hearing aids closely approximated an NAL NL-2 target as verified by real-ear aided responses (REAR).
An age effect was not apparent, as patient age ranged from 8 years to 82 years, and no similarities of occupation or level of education/income were apparent. There also appeared to be no relationship between time spent in noisy environments and normal bilaterally aided scores on the QuickSIN, contrary to the work of Olsen et al28 Saunders et al29 similarly questioned the use of an auditory training program for improving adult-patient speech perception in noise.
It is unfortunate so few subjects in a 2-year period were able to achieve a normal bilaterally aided score on the QuickSIN and may therefore avoid unfavorable brain resource reallocation, show improved cortically evoked latencies, have more appropriate distribution of regional glucose metabolism of both the IC and PAC, and show evidence of improved cognitive function in several domains. There are currently 831 patients in this clinic’s medical database, with unaided SII values between .50 and .75, yet only 63 of 831 patients scored normally on the bilaterally aided QuickSIN.
Fortunately, Glick and Sharma2 showed that subjects who had scores that approximate normal bilaterally aided QuickSIN scores displayed some cognitive benefit and evidenced some decreased brain resource reallocation.
Summary
Based on these findings, several clinical observations are offered:
- The hearing care professional should assure that sufficient audibility is provided by whatever brand, model, or age of hearing aid is utilized, as confirmed by real-ear aided measures and well-researched targets.
- Regularly scheduled follow-up appointments are necessary to maintain sufficient real-ear aided audibility over time by managing plugged hearing aid receivers/microphones, impacted cerumen, or changing hearing thresholds.
- Aided speech-in-noise scores should be routinely obtained to identify the difficulty each patient might experience in noisy environments, and identify those at greatest risk for cognitive resource reallocation and less favorable cognitive outcomes.
- External microphones should be provided for those who score poorly on such tests yet still must function in noisy environments. Considerable research supports use of remote microphone devices to improve speech perception in noise.30-37
- Based on the important contribution of cognitive factors to good SIN scores, some estimate of a patient’s cognitive ability should be screened, as per Lin.38 While not endorsing any particular cognitive screening test, the National Institute on Aging notes the Mini-Cog is a 3-minute screening tool and is available in many languages. In their meta-analysis of various cognitive tests, Breton et al39 noted the Memory Alteration Test was time-efficient and had the highest sensitivity and equivalent specificity to the other tests in their analysis. While assessing cognitive status is not in the scope of practice for hearing care professionals, a good case is made by Beck et al40 that we should screen for cognitive dysfunction and educate people about the link between better communication through improved hearing and brain health.
- Time spent listening in noisy environments did not seem important for these 63 subjects, consistent with findings of Saunders et al.29
Finally, patients should be encouraged to address poor hearing with properly programmed, real-ear verified hearing aids sooner rather than later based on the evidence provided by Glick and Sharma2 and Speck et al,15 suggesting there may be an upper limit to reversing compensatory neural changes in cortical resource allocation.In the present study, none of the 63 subjects had waited until their hearing loss exceeded the mild-to-moderate hearing loss range before pursuing hearing aids.

Correspondence can be addressed to Dr Leavitt at: [email protected].
Citation for this article: Leavitt RJ, Flexer C, Clark N. Variables associated with attainment of normal scores on the bilaterally aided QuickSIN Test. Hearing Review. 2020;27(9):18-21.
References
1. Glick H, Sharma A. Cross-modal plasticity in developmental and age-related hearing loss: Clinical implications. Hear Res.2017;343:191-201.
2. Glick HA, Sharma A. Cortical neuroplasticity and cognitive function in early-stage, mild-moderate hearing loss: Evidence of neurocognitive benefit from hearing aid use. Front Neurosci. 2020;14:93.
3. Killion MC, Niquette PA, Gudmundsen GI. Development of a quick speech-in-noise test for measuring signal-to-noise ratio loss in normal-hearing and hearing-impaired listeners. J Acoust Soc Am. 2004;116(4):2395.
4. Lin FR, Ferrucci L. Hearing loss and falls among older adults in the United States. Arch Intern Med.2012;172(4):369-371.
5. Jiam N T-L, Li C, Agrawal Y. Hearing loss and falls: A systematic review and meta-analysis. Laryngoscope.2016;126(11):2587-2596.
6. Lin F, Yaffe K, Xia J, et al. Hearing loss and cognitive decline in older adults. JAMA Intern Med. 2013;173(4):293–299.
7. Nixon GK, Sarant JZ, Tomlin D. Peripheral and central hearing impairment and their relationship with cognition: A review. Int J Audiol.2019;58(9):541-552.
8. Genther DJ, Betz J, Pratt S, et al. Association between hearing impairment and risk of hospitalization in older adults. J Am Geriatr Soc. 2015;63(6):1146-1152.
9. Yang M, Chen H-J, Liu B, et al. Brain structural and functional alterations in patients with unilateral hearing loss. Hear Res. 2014;316:37-43.
10. Li W, Li J, Xian J, et al. Alterations of grey matter asymmetries in adolescents with prelingual deafness: A combined VBM and cortical thickness analysis. Restor Neurol Neurosci. 2013;31(1):1-17.
11. Cacciatore F, Napoli C, Abete P, Marciano E, Traissi M, Rengo F. Quality of life determinants and hearing function in an elderly population: Osservatorio Geriatrico Campano Study Group. Gerontol. 1999;45(6):323-328.
12. Huang C-Q, Dong B-R, Lu Z-C, Yue J-R, Liu Q-X. Chronic diseases and risk for depression in old age: A meta-analysis of published literature. Ageing Res Rev. 2010;9(2):131-141.
13. Sinha UK, Hollen KM, Rodriguez R, Miller CA. Auditory system degeneration in Alzheimer’s disease. Neurol.1993;43(4).
14. Peelle JE, Troiani V, Grossman M, Wingfield A. Hearing loss in older adults affects neural systems supporting speech comprehension. J Neurosci. 2011;31(35):12638-12643.
15. Speck I, Arndt S, Thurow J, et al. 18 F-FDG PET imaging of the inferior colliculus in asymmetric hearing loss. J Nuclear Med. 2020;61(3):418-422.
16. Society of Nuclear Medicine and Molecular Imaging. High-resolution PET/CT imaging assesses brain stem function in patients with hearing impairment. https://hearingreview.com/hearing-products/implants-bone-conduction/cochlear-implants/high-resolution-pet-ct-imaging-assesses-brain-stem-function-in-patients-with-hearing-impairment. March 30, 2020.
17. Carhart R, Jerger JF. Preferred method for clinical determination of pure-tone thresholds. J Speech Hear Dis.1959;24(4):330-345.
18. Acoustical Society of America. ANSI/ASA S3.5-1997 (R2017). American National Standard Methods for Calculation of the Speech Intelligibility Index. American National Standards Institute, New York:1997.
19. American Speech-Language-Hearing Association. Determining threshold level for speech [Guidelines].1998.
20. Olsen WO, Noffsinger D. Comparison of one new and three old tests of auditory adaptation. Arch Otol.1974;99(2):94-99.
21. Peterson GE, Lehiste I. Revised CNC lists for auditory tests. J Speech Hear Dis. 1962;27(1):62-70.
22. Cox RM, Alexander GC, Taylor IM, Gray GA. The contour test of loudness perception. Ear Hear.1997;18(5):388-400.
23. McArdle RA, Wilson RH. Homogeneity of the 18 QuickSIN lists. J Am Acad Audiol. 2006;17:157-167.
24. Keidser G, Dillon HR, Flax M, Ching T, Brewer S. The NAL-NL2 prescription procedure. Audiol Res.2011;1(1S).
25. Acoustical Society of America (ASA). ANSI/ASA S3.7-1995 (R2008):Method for Coupler Calibration of Earphones. American National Standards Institute, New York, NY:2003.
26. Leavitt RJ, Vossler CB, Knowles LR. Cost-effective pricing for hearing aids and related audiological services. Hearing Review.2011:18(12):28-35.
27. Cox RM, Johnson JA, Xu J. Impact of advanced hearing aid technology on speech understanding for older listeners with mild-to-moderate, adult-onset, sensorineural hearing loss. Gerontol. 2014;60(6):557-568.
28. Olson AD, Preminger JE, Shinn JB. The effect of LACE DVD training in new and experienced hearing aid users. J Am Acad Audiol. 2013;24:214-230.
29. Saunders GH, Smith SL, Chisolm TH, Frederick MT, McArdle RA, Wilson RH. A randomized control trial: Supplementing hearing aid use with Listening and Communication Enhancement (LACE) auditory training. Ear Hear.2016;37(4):381-396.
30. Kreisman B, Crandell C. Behind-the-ear FM system: Effects on speech perception in noise. J Educ Audiol.2002;10: 21-25.
31. Tharpe A, Ricketts T, Sladen D. FM Systems for Children with Minimal to Mild Hearing Loss. In: Fabry D, Johnson CD, eds. ACCESS: Achieving Clear Communication Employing Sound Solutions-2003:Proceedings of the First International Conference in Print and on CD-ROM. Phonak AG;2004:191-197.
32. Lewis MS, Crandell CC, Valente M, Horn JE. Speech perception in noise: Directional microphones versus frequency modulation (FM) systems. J Am Acad Audiol.2004;15:426-439.
33. Lewis MS, Hutter M, Lilly DJ, Bourdette D, Saunders J, Fausti SA. Frequency-modulation (FM) technology as a method for improving speech perception in noise for individuals with multiple sclerosis. J Am Acad Audiol.2006;17(08):605-616.
34. Johnston KN, John AB, Kreisman NV, et al. Multiple benefits of personal FM system use by children with auditory processing disorder (APD). Int J Audiol.2009;48(6):371-383.
35. Umat C, Mukari SZ, Ezan NF, Din NC. Changes in auditory memory performance following the use of frequency-modulated system in children with suspected auditory processing disorders. Saudi Med J.2011;32(8):818-824.
36. Hornickel J, Zecker SG, Bradlow AR, Kraus N. Assistive listening devices drive neuroplasticity in children with dyslexia. Proc Natl Acad Sci (PNAS). 2012;109(41):16731-16736.
37. Koohi N, Vickers D, Chandrashekar H, Tsang B, Werring D, Bamiou D-E. Auditory rehabilitation after stroke: Treatment of auditory processing disorders in stroke patients with personal frequency-modulated (FM) systems. Disabil Rehabil. 2017;39(6):586-593.
38. Dr Frank Lin details consequences of age-related hearing loss and future avenues at ADA Convention Keynote. Hearing Review. https://hearingreview.com/inside-hearing/research/dr-frank-lin-details-consequences-age-related-hearing-loss-future-avenues-ada-convention-keynote. Published November 11, 2014.
39. Breton A, Casey D, Arnaoutoglou N. Cognitive tests for the detection of mild cognitive impairment (MCI), the prodromal stage of dementia: Meta‐analysis of diagnostic accuracy studies. Int J Geriat Psych.2019;34(2):233-242.
40. Beck DL, Weinstein BR, Harvey MA. Dementia screening: A role for audiologists. Hearing Review.2018;25(7):36-39.
ALSO SEE the interview with Anu Sharma by Douglas Beck in the February 2020 HR,41 as well as Dr Sharma and Hannah Glick’s article, “Cortical Neuroplasticity in Hearing Loss: Why It Matters in Clinical Decision-Making for Children and Adults” in the July 2018 HR.42
41. Beck DL. How might the brain change when we reintroduce sound? Interview with Anu Sharma, PhD. Hearing Review. 2020;27(4).
42. Sharma A, Glick H. Cortical neuroplasticity in hearing loss: Why it matters in clinical decision-making for children and adults. Hearing Review. 2018;25(7):20-24.




